Translate this page into:
Polynucleotides and polydeoxyribonucleotides in dermatology – A narrative review
*Corresponding author: Gulhima Arora, Department of Dermatology, Mehektagul Dermaclinic, New Delhi, India. gulhima@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Arora G. Polynucleotides and polydeoxyribonucleotides in dermatology – A narrative review. J Cutan Aesthet Surg. doi: 10.25259/JCAS_65_2025
Abstract
Polynucleotides (PNs) and polydeoxyribonucleotides are deoxyribonucleic acid (DNA)-derived bioproducts mostly sourced from salmon gonads. They are unique as they are biocompatible, inert, and have a strong regenerative potential with studied effectiveness in various dermatological indications. They act by binding to the adenosine A2 receptors and through the salvage pathway of DNA synthesis and cause several pharmacological actions such as immune modulation, inhibiting inflammation and melanogenesis, promoting angiogenesis, and acting as biostimulators by causing cell proliferation. Although their positioning as drugs in the regenerative segment is promising, more controlled studies are needed to bridge the gaps in evidence-based use of PNs and to address certain critical issues. This narrative review highlights key aspects of the present position of these bio-drugs and places them as pivotal inserts for skin and tissue regeneration, laying stress on the evidence, clinical gaps, ethical considerations, regulations, and alternative sourcing.
Keywords
Polydeoxyribonucleotides
Polynucleotides
Regenerative medicine
Skin boosting
INTRODUCTION
Polynucleotides (PNs) and polydeoxyribonucleotides (PDRN) represent an exciting advancement in pharmacotherapy with a robust regenerative potential as they can modulate gene expression. They are deoxyribonucleic acid (DNA)-derived drugs with tissue repair, therapeutic angiogenesis, anti-inflammatory, and immune-modulating properties. Their sourcing, molecular structure, and weight place them as therapeutic agents, which can be used for numerous indications due to their unique mechanism of action. The recent significant attention they are receiving and their involvement in various dermatological indications is also due to their safety profile and biocompatibility.
BIOLOGY OF PN AND PDRN
Nucleotides are chains of organic molecules containing monomeric units of nitrogen and phosphate bases and a sugar molecule. The chains may be oligonucleotides (short chains with mostly <30 monomeric units) or PNs (longer chains).
These form the building blocks of the genetic material, DNA or ribonucleic acid (RNA), which are also PNs.
The sugar molecule in DNA is deoxyribose, whereas, in RNA, it is ribose, both being pentose sugars. The nitrogenous bases may be purines (adenine and guanine) or pyrimidines (thymine, cytosine or uracil). The pairing of one purine with a pyrimidine base forms the genetic code. The sugar and nitrogenous bases together form a nucleoside, to which one or more phosphate groups are attached to form a nucleotide [Figure 1].
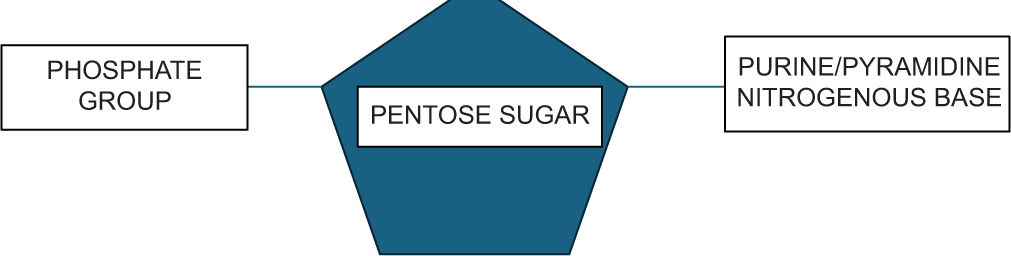
- Monomeric unit of polynucleotide.
PNs are biopolymers which are present in every cell. They are chains of nucleotides which provide the genetic code to a living organism, modulate gene expressions and bring about downstream effects such as modulation of inflammation and immune responses, wound healing, and regeneration by stimulating cell proliferation and differentiation.
Nucleotides are only sparingly soluble in water. Formulation methods to make them more soluble must be done in a controlled way to prevent degrading the molecule. They are thermoresistant and absorb ultraviolet (UV) light in the region of 260 nm.1 The addition of hyaluronic acid (HA) to PNs has been shown to increase the proliferative capacity of PN.2
CHEMISTRY OF PNS AND PDRNS
The nucleotides present in PN may have ribose or deoxyribose as the sugar moiety, as opposed to PDRN, where it is only deoxyribose. PN may be sourced from DNA or RNA, but usually are DNA-derived and are longer fragments of the same. The PDRN extracted from salmon fish have shorter, linear, and single-stranded chains with a double helix spatial structure, and the length of the molecule has 50–2000 base pairs (bp).3,4
PN is a broader category under which PDRN is a subset. The molecular weight of PN and PDRN is typically between 50 and 1500 kilo Daltons (kDa); however, recent studies have included DNA fragments from 1 kDa up to 10,000 kDa.5 Although PN and PDRN have similar molecular effects, and the terms are used interchangeably in scientific literature, it is to be understood that they are different polymers and bioequivalent drugs. To retain their individual identity, the “Marques Polynucleotide Cutoff ” was proposed to differentiate PN having molecular weights ≥1500 kDa and PDRN <1500 kDa.5 Differences in their physicochemical characteristics due to different molecular weights can lead to differences in product properties. Both are highly biocompatible due to their origin from natural sources.
PN “cells” or “shapes” are pentagonal or hexagonal with a slight difference in the sizes. The diameter of these is around 3–5 um.6 The substance of PN is shown to have intricately placed cells, with thin walls as seen on scanning electron microscopy. The cells are arranged in a tessellated pattern, without any gaps. These ultrastructural patterns place PNs uniquely, as their thin walls make them amenable to be used as slow-release encapsulated drugs, and the ability to modulate the elasticity of the extracellular matrix (ECM) due to their efficient perimeter-area ratio and unique mechanical properties.6 Defibrotide, another natural DNA drug, is sourced from porcine intestinal mucosa and has a similar mechanism of action to PNs. However, due to having different isolation sources, molecular weights and manufacturing techniques, it has different pharmacological properties.7 An example in this regard is that PDRN stimulates angiogenesis, whereas defibrotide inhibits it. Regadenoson is another DNA drug approved by the US Food and Drug Administration as a pharmacological stress agent used for myocardial perfusion imaging, and just like PDRN, which also binds to adenosine 2A (A2A) receptors and has similar but not same pharmacological properties.
PN acts by providing building blocks for nucleic acid synthesis needed for cellular proliferation and repair. It thus provides slower but long-lasting effects. PDRN acts directly on regeneration and repairing damaged DNA. It increases fibroblast activity, induces angiogenesis, and is anti-inflammatory. PN provides long-lasting structural support thus providing a broader skin rejuvenation. Its actions are more robust, cause enhanced collagen synthesis and have a stronger inflammatory response. PDRN causes more targeted action [Table 1].6,8
| PNs | PDRNs | |
|---|---|---|
| 1. | Longer fragments | Shorter chains typically with 10–30 nucleotides |
| 2. | Molecular weight ≥1500 kDa | Molecular weight <1500 kDa |
| 3. | Higher commercial price | Less than PN |
| 4. | Testes (broader source) | Sperm Cell |
| 5. | More potent | Less potent |
| 6. | Less stable due to longer chains | More stable |
| 7. | Distinct scaffold structure | Not present in PDRN |
| 8. | Larger molecule, hence used for larger areas which need correction | Smaller size, therefore used for smaller areas such as the infraorbital region |
| 9. | Longer lasting effects | Effects are shorter lasting |
| 10. | Sustained regeneration | Rapid healing and rejuvenation |
kDa: kilo Daltons, PN: Polynucleotides, PDRN: Polydeoxyribonucleotides
HISTORY OF PN
An early publication in 1963 highlighted the possible therapeutic effect of synthetic PNs on mammalian cells, which were synthesized by PN phosphorylase isolated from Micrococcus lysodeikticus.9
PN/PDRN were first used as therapeutics before their role was explored in medical esthetics. PDRN is a registered DNA-derived propriety drug and was used for indications such as wound healing, chronic venous ulcers, and osteoarthritis knee.10 In the 1990s, there were published studies on the benefits of the use of PDRN in cervical ectropion, post-cauterization re-epithelialization, and wound healing due to its ability to cause fibroblast proliferation.5,8 This property led to its use as an anti-aging drug.
Initial studies on human placenta extracted PNs were conducted on human knee skin fibroblasts. Research and development by the company Albert David Limited led to an injectable drug called Placentex®. This is now also available as a gel.
Newer sources and indications are now being explored.
PHARMACOKINETICS OF PDRN
Following an intraperitoneal injection of 8 mg/kg of PDRN in a rat, a bioavailability of 90% was documented with peak levels reaching in 1-h post-injection. The half-life was measured to be 3 h and not affected by the dose of administration. However, the pharmacodynamic transduction effects last longer. The drug is found free in plasma and is distributed to those organs which have greater vascularity. It is a prodrug that is broken down by plasma and membrane-bound DNA nucleases into oligo and mononucleotides, which bring about pharmacological activity. The liver does not metabolize it. Around 65% of the drug is eliminated in the urine and some amount in the feces. This profile matches the pharmacokinetics seen in humans after an intramuscular injection of PDRN.11
The shelf life of PN/PDRN products is 3 years from the date of manufacture when stored at 2–8°C. Since it is a biodrug, immediate use of the opened vial and discarding of the remaining product is recommended.12
SOURCING OF PN AND PDRN
Although PDRN has been sourced from the human placenta, PNs for medicinal purposes are now commonly sourced from salmon fish. PN is obtained from a broader source, the gonads of the salmon fish, whereas PDRN is sourced from sperm cells. The testicles of adult male salmon are filled with sperm and the DNA content is 7.5% of its wet weight.4 Salmon spermatozoa as a source is unique as it is immune-privileged due to high purity without the additives of proteins or lipids and has a high content of DNA which is similar in structure to human DNA, thus being inert and biocompatible. The fish commonly used are the salmon or rainbow trout (Oncorhynchus mykiss) or chum salmon (Oncorhynchus keta).13 Acipenser sinensis (Chinese Sturgeon) is another source for PDRN and PN.
PDRN and PN sourced from plants, phyto PDRN, have also been found to have similar pharmacologic effects with the advantage of sustainable and ethical sourcing. The adventitious roots of Panax ginseng (Korean ginseng) are one such source of PDRN, which was purified using microfluidization after suspension-cultivation through tissue culture.14 PDRN has also been sourced from red alga (Porphyra spp.) and shown to have proliferative effects on dermal fibroblasts.15
EXTRACTION PROCESS
A good manufacturing practice (GMP) facility and following strict guidelines and regulations for extraction are mandatory. Strict control measures to isolate and purify the drug must be in place to prevent contamination. Rigorous purification methods including the use of high temperatures, are employed to isolate DNA from the sources allowing for a recovery of 95% or more of the purified drug.13,14 It is essential that the extraction process ensures a safe, biocompatible and efficacious product with the least immunological potential.
The method of extraction from fish includes (a) collection of sperm from the salmon fish – adopting sterile conditions for isolation from mature, male fish. (b) Lysis of cells – involves using gentle buffers to break the sperm cells to release intact nucleotides. (c) Filtration and centrifugation – to remove cellular debris and contaminants. (d) Removal of endotoxins – using resins. (e) Chromatography – high-performance liquid chromatography is used to purify the contents further and separate the PDRNs according to their size, hydrophobicity or charge. (f) Sterilization – to prevent contamination is done using methods such as heat irradiation or filtration. (g) Freeze-drying – to enable long-term storage of the product by converting it into a stable powder form.3
MECHANISM OF ACTION
Both PN and PDRN may have overlapping mechanisms of action, but as stated earlier, PN acts by providing the nucleotides for DNA/RNA synthesis, whereas PDRN acts by directly causing healing and tissue regeneration [Figure 2].
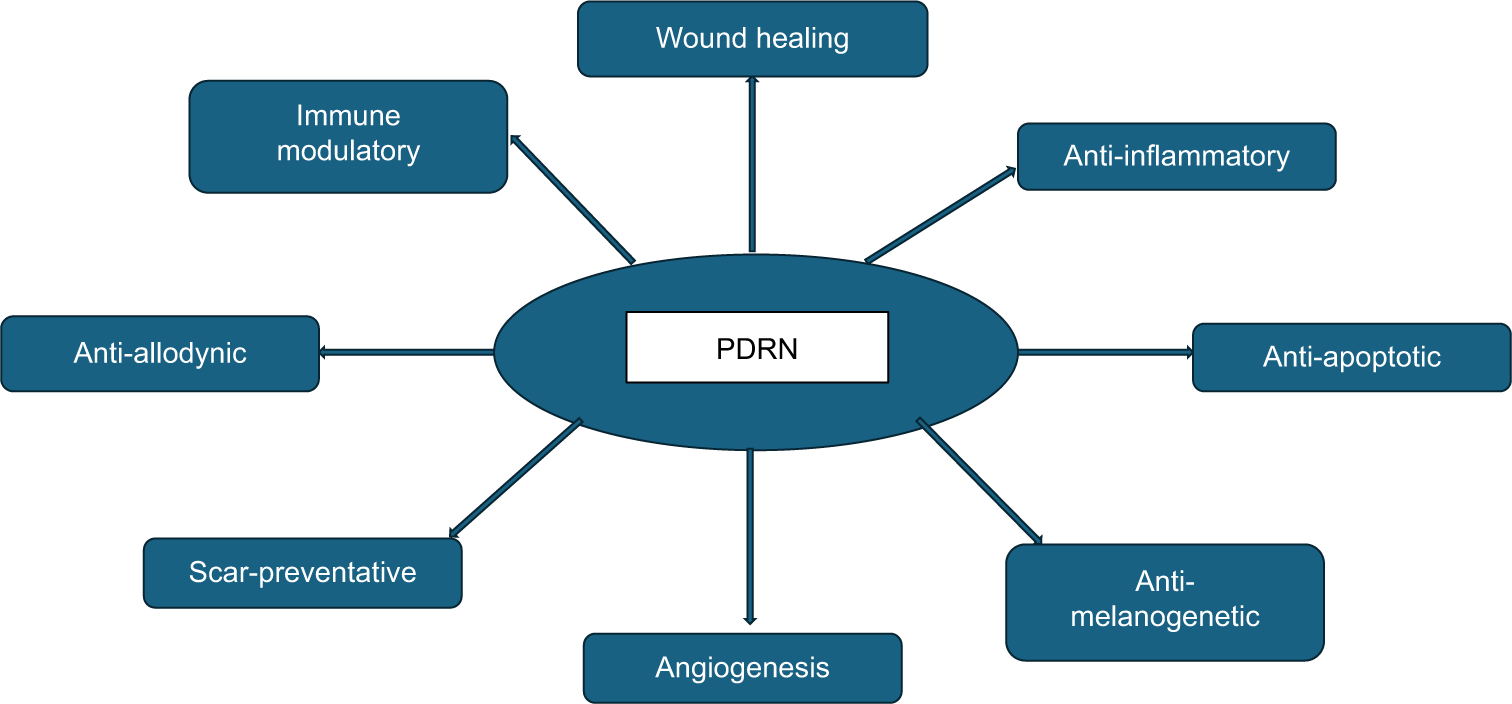
- Pharmacological actions of polydeoxyribonucleotides pertaining to dermatology. PDRN: Polydeoxyribonucleotides.
The multiple pharmacological effects brought about by PDRN are due to the binding of adenosine in the oligo and mononucleotides of PDRN to A2A receptors and its participation in the salvage pathway.11,13 Binding to A2A receptors is unique to this natural DNA-derived molecule of this specific molecular weight, between 50 and 1500 kDa. Other PDRN bioequivalent drugs or DNA-derived molecules which have molecular weights outside of this range, do not bind to this receptor, and should not be called PDRN.11 There are many in vitro studies on cultured fibroblasts, chondrocytes, and osteoblasts, that support the different pharmacological actions of PDRN.2,16 The A2A receptor antagonist, 3,7 – Dimethyl-1-propargyl xanthine, negates the effects of PDRN, supporting this evidence of the molecule preferentially acting on this receptor.11 The A2A receptor agonist CGS-21680 has shown to have similar effects to PDRN on collagen synthesis in vitro.13 PDRN thus acts as a prodrug, which acts by releasing its components, the nucleotides, nucleosides, and nitrogenous bases, which bind to the A2A receptor. The salvage pathway is an energy-saving one that ensures the synthesis of DNA by providing the building blocks in the form of nucleotides and bases when de novo synthesis is not possible. This situation arises when during periods of extreme stress due to reactive oxygen species (ROS) or ischemia.17
The mechanisms of action are outlined in Table 2. Phyto PDRN also acts through this receptor.14
| Mechanism of action | Effect | Clinical indication | |
|---|---|---|---|
| I | Binding to A2A receptor | ||
| All actions are via signaling pathways. Inhibition of MAPK and NFκB. | Increase in anti-inflammatory cytokines like IL-10, decrease in TNF-α, IL-6, and IL-12 | Useful in inflammatory conditions | |
| Protect cells from UV damage | |||
| Improves fibroblast viability, migration, and proliferation | Wound healing | ||
| Downregulation of HMGB-1 | ECM production, Collagen and elastin synthesis | Improvement in skin elasticity, radiance, hydration and texture | |
| Increased expression of Vascular Endothelial Growth Factor | neovascularization | Angiogenesis | |
| Cyclobutene pyrimidine dimer repair | Protection against UVB damage | Anti-aging properties | |
| Inhibition of MMP 1 | Increase in collagen synthesis, inhibits elastase | Antiaging effects, skin texture improvement, improves skin elasticity | |
| Inhibition of FLI1 mRNA and consequent upregulation of CTGF | Increase in collagen | Antiaging properties, improvement in skin texture | |
| Inhibition of JAK/STAT pathway via upregulation of SOCS3* | Decreased LDH | Prevents neuronal injury | |
| Inhibition of tyrosinase, MITF, TRP-1, TRP-2 | Inhibits melanogenesis | Pigmentary conditions | |
| Inhibition of ERK and p38 MAPK pathways | Inhibition of NO and NO synthase protein, proliferation of dermal fibroblasts | Anti-inflammatory effects on Escherichia coli LPS-stimulated RAW 254.7 macrophages promotion of collagen synthesis, wound healing | |
| Enhances Wnt1 and β-catenin mRNA expression | Modulates signaling | Hair restoration, improving hair count and thickness | |
| Activation of transcription factors such as NFκB, CREB, and HIF-1 | Cellular response to hypoxia, inflammation and oxidative stress | Tissue regeneration | |
| II | Salvage Pathway | Provides nucleotides and nucleosides for DNA synthesis during periods of stress or hypoxia when de novo DNA synthesis is hampered | Reverses UV damage and damage due to ROS |
| III | Increase proliferation of pre-adipocytes | Increase source of stem cells | Tissue regeneration, hair restoration |
| IV | Dermal priming effect | Enhances the effect of other complimentary treatments | |
| V | Increase effect of filaggrin, fibronectin, Ki-67, Bcl-2 genes^ | Proliferation of keratinocytes and fibroblasts. | Barrier repair, wound healing |
A2A receptors are present in the cell membrane of cells of several human tissues; however, they are highly concentrated in the immune cells, which highlights their role in inflammation.18 Adenosine receptors are of three types A1, A2A, A2B, and A3. Signal transduction through A2A receptor binding is responsible for modulating inflammation, ischemia, cell proliferation, and differentiation and angiogenesis.14 Receptor-binding causes the release of cyclic adenosine monophosphate through stimulation of Gs protein, which inhibits signaling pathways such as mitogen-activated protein kinase and nuclear factor kappa beta (NF -κB) and stimulates the transforming growth factor-beta/suppressor of mothers against decapentaplegic signaling pathway.19 The mechanisms of action of PN are similar.6 The net effect of these interactions reverses the actions of ROS generated through UV ray exposure or mitochondrial aerobic metabolism and leads to a decrease in the pro-inflammatory cytokines and matrix metalloproteinases.
The following mechanisms of action have been elucidated with both, in vitro research and confirmed in vivo on humans as well [Figure 3].

- An outline of the two main pathways of action of polydeoxyribonucleotides. PDRN: Polydeoxyribonucleotides.
Anti-inflammatory effects
The reduction in inflammatory cell infiltrate and pro-inflammatory cytokines has led to PDRN reflecting its usefulness in various acute and chronic inflammatory conditions. PDRN reduces the recruitment of leucocytes and the production of toxic oxygen metabolites in neutrophils.18 Western blot analysis has confirmed that it decreases the levels of tumor necrosis factor alpha (TNF-α)x and increases the levels of vascular endothelial growth factor (VEGF), nitric oxide synthase, and the ratio of nitrous oxide is to nitric oxide (NO2/NO3). The M1-activated phenotype of macrophages is converted into the anti-inflammatory M2 type.18
Tissue regeneration
PDRN aids in wound healing by increasing fibroblast proliferation, migration, and viability with no cytotoxic effects.20 Tissue regeneration due to impaired activity due to pathological processes such as malignancy, oxidative stress, infections, or vascular diseases can be achieved with the use of PDRN. It reverses the deleterious effects of UV ray damage caused by increasing the expression of matrix metalloproteinase 1 (MMP-1) and ROS, leading to DNA damage.13
ECM modulation
PDRN exhibits collagen synthesis properties by its proliferation effect on fibroblasts and inhibition of high mobility group box nuclear proteins.21 It decreases the amounts of MMPs in inflamed, non-healing skin. It is responsible for cell proliferation, migration, and remodeling. An interesting study concluded that mid-molecular weight PDRN (50–1500 kDa) was better than low or high-molecular weight ones and promoted faster collagen synthesis and better quality of wound closure.22
Collagen synthesis
PDRN increases collagen synthesis in a dose-dependent manner, a property not seen with HA.13 Studies on human dermal fibroblasts have revealed that it inhibits the friend leukemia integration-1 messenger RNA (mRNA) in the nucleus and correspondingly increases the connective tissue growth factor mRNA, leading to a net increase in collagen.
Inhibition of melanogenesis
PDRN decreases melanin synthesis significantly in a dose-dependent manner. It inhibits the melanogenic genes and tyrosinase enzymatic activity. It also inhibits the proteins Tyrosinase-Related Protein (TRP)-1 and TRP-2.13
Angiogenesis
Macrophages are stimulated to produce VEGF, the main growth factor needed for angiogenesis and tissue regeneration and wound healing. VEGF also mitigates inflammation, thus aiding in wound healing. A2A receptors on the toll-like receptor-activated macrophages favor a switch from the pro- to anti-inflammatory cytokine release and the production of VEGF and interleukin -10.18 This was confirmed by CD31 analysis and an increase in microvessel density. Transglutaminase-II and angioprotein levels are also increased which is suggestive of angiogenesis.13
Barrier repair
PDRN helps to restore a damaged skin barrier due to the expression of certain genes such as filaggrin and through immune signalling.14 This indication helps in the treatment of sensitive skin.
The mechanisms of action are elaborated in Table 2.6,11,13-16,18,21,23-25
MODES OF DELIVERY
PDRN is obtained as a white powder or a fibrous solid. It is reconstituted and delivered through various modes.4
The drug is mostly injected intradermally or delivered into the skin through microneedling. Topical anesthesia can be used to numb the skin. Injections are made using 32–33 G needles using serial puncture technique usually, mesoguns or cannulae. Injections allow for a deeper penetration. The depth of microneedling is dependent on the indication and anatomical site where the drug needs to be delivered.
Delivery of PDRN/PN post ablative lasers such as carbon dioxide or erbium can also be done.13 This method may be used to help with lesser downtime and faster healing, or as a mode of drug delivery through the channels created, combining the two treatments.
Topical formulations of PDRN/PN are gaining attention due to their non-invasiveness. The different formulations available are creams, serums, lotions, essences, and masks. PDRN healing gels are available for use after an ablative procedure.18 Hydrogel dressings have been used for diabetic ulcers.26 Topicals, however, may have limited penetration and, hence, are less efficacious than injections. They may be useful as maintenance treatments and in improving skin hydration.
PDRN/PN are also delivered through electroporation and iontophoresis. These methods may have the advantage of having no downtime. An oral spray formulation has been used for oral mucositis.27
PN-containing devices are used as temporary fillers (intradermal gels) due to the viscoelastic properties of PNs. These act as bioreactivators and improve the skin health and cause tissue regeneration.28 The PN Highly Purified Technology TM (PN-HPT TM) device contains 15 mg/2 mL of intradermal gel.28
A study by Morganti et al. concluded the role of PN, a biodegradable fiber containing non-woven tissue, as a delivery system for cosmeceutical delivery. It was seen to improve the bioavailability, skin penetration, and controlled release of the cosmeceutical ingredients and inhibit any allergic reactions to the product. Many topical products such as moisturizers, antioxidants, and vitamins have been delivered using PN.6
PROTOCOL FOR ADMINISTRATION
There is no standardized protocol for administering PN/PDRN. Most studies have revealed a treatment plan wherein the drug is delivered once in 2–4 weeks and 3–4 sessions of the initial phase treatment are done. Maintenance sessions are scheduled periodically. Results are apparent in 4–8 weeks and improve over 3–6 months.13
COMBINATION TREATMENTS
PDRN treatments can be combined with other esthetic treatments depending on the indication that they are being used for. Lasers, light-based treatments, and energy-based devices are all used in combinations both for skin rejuvenation as well as for hair restoration.13 HA injectables are also combined with PDRN for use in volume restoration or skin health boosting. Interestingly, it has been found that the addition of even as low as 1mg/ml of HA to PDRN increases its activity by up to 20%.2 Medifacials incorporating PDRN skin masks may add to the benefit of the procedure. Home care skin products containing PDRN can be prescribed with any of the above treatments.
SAFETY PROFILE OF PN/PDRN
Individuals with hypersensitivity to fish or fish products should be cautious with the use of these DNA drugs sourced from salmon as they may lead to an allergic reaction.3 However, not all patients with fish allergy develop reactions to PN/PDRN. These products are purified by filtration during extraction. Prior patch testing with the drug may help identify such patients.
A few studies that have elucidated the safety profile of PDRN have shown that it is a well-tolerated and safe drug, with no related complications. Several studies have shown that this biodrug is non-toxic. No data however is present on the long-term evaluation of the drug. No mutagenic potential of the drug has been reported so far. However, since it is a biodrug and it increases VEGF and hypoxia-inducible factor-1, some concerns about its theoretical mutagenic potential have been raised.29
Most of the side effects are localized due to the mode of delivery. A minimal downtime with redness and swelling may be present after intradermal injections or microneedling for 1–3 days. Mild discomfort may be noted during the injectable treatment which can be alleviated with the use of ice packs and topical numbing. A few bumps may be present post-treatment, lasting a few hours to a day or two.3 The use of intradermal gel devices may cause burning for up to 12 h after the injections, bruising, and small hematomas rarely.28 In a consensus statement, it has been recommended to avoid exposure to sun lamps and sunlight for 48 h post-PN/PDRN treatment.28
The procedure should be performed with aseptic precautions in place to avoid infection of the treated area.
Both, PN, and PDRN can cause temporary histamine reactions. The use of PN/PDRN in pregnancy and lactation is not recommended due to a lack of studies.
REGULATORY CHALLENGES
Analytical methods for PDRN testing should be in place. Optimal operating conditions and quality control must be maintained from isolation to the final product, using a GMP facility. Stability reports with stability data must be in place to ensure that the drug remains stable in different storage conditions. All regulatory compliance documents complying with international and regional regulations should be present. Challenges arise due to inadequate operating protocols and structured guidelines for sourcing and manufacturing.30
Limitations
Although the use of PDRN and PN has revolutionized the approach to treatment of various dermatological conditions, yet it is a treatment with limitations [Table 3]. The isolation, extraction and purification of the biodrug is complicated and makes it expensive. Isolation from salmon fish is dependent in the breeding season. Ethical concerns of using fish as a source of the PNs cannot be ignored.
| 1. Expensive drugs |
| 2. Ethical considerations of isolation from salmon fish |
| 3. Complicated extraction and purification |
| 4. Few standardized protocols for use |
| 5. Alternate sources such as plants, lack evidence-based studies |
| 6. Inter-batch variability from the same source |
PN: Polynucleotides, PDRN: Polydeoxyribonucleotides
A systematic review submitted certain considerations for the use of PNs in regenerative and esthetic medicine. With a cohort of 750 participants and 360 studies, it concluded that though the potential of PNs is significant, yet, current evidence for their use is fraught with inconsistencies and caution needs to be exercised to ensure that patient safety is not compromised.30
A systematic review conducted in 2024 to evaluate the scientific basis and empirical evidence of the use of PDRN revealed several shortcomings for its use.30 More controlled trials and extrapolation of the in vitro findings to translational medicine need to be undertaken. A standardization of doses, mode of delivery of the drug, exact downstream effects, safety profile, drug interactions, sourcing, extraction, and storage of the drug, all need to be elucidated in order to accept it as a therapeutic agent with established quality and reliability.
INDICATIONS OF PDRN IN DERMATOLOGY
PDRN and PN are products that typically fit into the realm of medical esthetics, as they are increasingly being used for therapeutic indications.31,32 It is a biodrug, hence with more potent mechanisms of action. Patients are counseled about the cost:
Benefit ratio
A few patients enquire about the treatment after gaining awareness from peers or the media.
Esthetic indications
PDRN is used in esthetic medicine to reduce wrinkle depth, treat fine lines, for improving skin texture and overall skin health. Skin elasticity improves with its use, giving it a more youthful appearance.6,13
Skin boosting
In 2014, the first PN to be used as a skin booster was Rejuran®. PN and PDRN are being extensively used for skin-boosting due to certain unique properties such as being natural biopolymers, having high water-attracting and retaining capacity and biostimulation of dermal fibroblasts, thus making them score over small particle size HA for skin-boosting.33,34 A dose-dependent increase in collagen synthesis is seen unlike that seen with HA.35
Pigmentary conditions
Alopecia
Both androgenetic alopecia and female pattern hair loss have been treated with PDRN. It has been shown to increase the hair count and thickness in studies.37,38 Combination treatments of platelet rich plasma (PRP) with PDRN induce greater improvement in hair thickness compared with PDRN alone but not in hair counts.39 A PN-based gel containing 7.5 mg/mL has also been used for improvement in pattern hair loss in both males and females. It has been shown to decrease hair shedding and improve hair thickness.40
Wound healing
The use of PDRN has also been established in cases of pressure ulcers when injected and used as a gel.41 Intramuscular and perilesional subcutaneous injections of PDRN 5 times a week and twice a week for 8 weeks have been shown in a double-blind, randomized controlled trial to give significantly higher healing compared with placebo in difficult-to-heal diabetic ulcers.42 A study comparing the wound healing effects of PDRN injection and PDRN cream from O. keta and PDRN injection from O. mykiss showed that wound healing effects were similar in the injection groups from both sources but less with the cream.43
Scars
Several studies have reported the positive effects of PDRN in scar treatment. Intralesional and subcutaneous PDRN injections (5.625 mg/3 mL vials) were injected once a month for 6 months along with topical PDRN and systemic nutraceutical treatment in a case of post-traumatic and post-surgical scar with significant functional and esthetic improvement.42
Skin grafts
PDRN significantly increases the percentage of reepithelialization of the donor sites of skin grafts. A double-blind, placebo-controlled, and randomized study concluded its trophic effect on skin explants.44
Miscellaneous
Nerve impingement conditions with radiating pain such as ganglion cyst laying pressure on sciatic nerve or carpal tunnel syndrome, have also been satisfactorily managed with PDRN.45,46
PDRN’s immunomodulatory effects have led to its use in lichen sclerosus. Long-standing remissions were noticed after eight sessions of intradermal PDRN injections when combined with daily topical corticosteroids. Improvement was seen in inflammation, atrophy, leukoplakia, and pigmentation.18,47
Oral mucositis post-radiation therapy has also successfully been treated with PDRN. Pain relief occurred within 2–3 days of starting treatment and the lesions attenuated to Grade 2 within the 1st week of treatment with a topical preparation.27
PDRN has shown to ameliorate imiquimod-induced psoriasis due to its NF-κB Pathway Inhibition and Wnt/β-Catenin signaling modulation.48
A recent potential application of PDRN has been highlighted in inducing fat browning for anti-obesity purposes.11
INDICATIONS FOR THE USE OF PN IN DERMATOLOGY
PN has also been used for similar indications like PDRN, for both esthetic and therapeutic indications.
Its effectiveness in skin rejuvenation of the face and body such as neck aging by improving skin thickness, improving skin elasticity, reducing pore siz e, improving skin tone, skin sagging, and wrinkling has been sustantiated.6,49 It has been used in cocktails with PRP, HA, and growth factors for skin boosting.6
Similar results are seen in Asian skins, which have thinner epidermis.50 In a Korean study, the top six indications for the use of PN were (in order of decreasing popularity), fine lines on the cheek, infraorbital fine lines, fine lines in the periorbital area, uneven texture of the facial skin, skin dryness, skin glow, and fine lines on the forehead.6
PN is increasingly being used as a filler material as it provides a more natural-looking result without any major risks or complications as it is bio inert. Proper patient selection is necessary for optimal results.51 PN-HPT TM is used intradermally for skin rejuvenation, revitalization and evening skin tone for both the face, and body. The neck, décolleté, and hands are the body areas that have been treated. These treatments have also been used in the scalp, eyebrows, and beard for normalization of follicular activity and hair growth.28 A concept called the “PN-HPT TM priming” is the use of PN to prepare the tissues for a stronger and faster response to a second revitalization stimulus. It prepares the tissues before combination with other treatments for both, esthetic treatments as well as wound healing.28,41
PN filler when compared with HA filler for treating periocular crow’s feet wrinkles showed the same result, but was found to last longer.6 When compared to polycaprolactone for the same indication, both showed improvement, but PN was faster to act and lasted longer with fewer side effects such as bruising and swelling.52
Hair growth and hair follicle revival is shown to increase with the use of PN-HPT TM, injected into the scalp once in 7–14 days for 4 weeks, followed by 4 more sessions 21– 30 days apart.28
A randomized, double-blinded controlled trial revealed that an adjuvant treatment with PN along with fractional laser led to a favorable outcome in wound healing. PN-based therapy has shown to improve scar visibility and improved skin texture across cases with post-traumatic, burn, or postoperative scars, including scars after open thyroidectomy.53,54
A prospective controlled study was conducted in a small population to study the effect of highly purified PN for the treatment of moderate to severe acne scars, and it concluded its effectiveness in the treatment.55 PN has also been useful in treating post-surgical atrophic scars.
Facial erythema due to inflammatory facial dermatoses such as rosacea, eczemas, or photoaging has been treated with PN in combination with lasers or microneedling radiofrequency and phototherapy. It has been found effective in protection of the skin barrier, improve skin hydration, vascular stabilization, and anti-inflammation.6,56
PN-HPT TM has been found useful for stretch mark treatment in combination with PN along with HA vials for injection.28 A medical device containing polyphosphoric acid, mannitol, HA, and PN-HPT was applied which intradermally showed improvement in 85% of patients with stretch marks.57
PN is a promising alternative for the treatment of periorbital rejuvenation with significant improvement in the under-eye area. It helps with laxity, wrinkling, and pigmentation. PN filler has been used and compared with non-cross-linked HA fillers for periorbital rejuvenation. The improvement in skin elasticity and hydration was better seen in the PN group, and so was the improvement in the pore volume and skin roughness. There were no adverse effects seen.58
Several studies have highlighted the effectiveness of PN for vulvo-vaginal rejuvenation. It ameliorates vaginal atrophy, vaginal dryness, and itching. It has been used as a filler with HA for labia majora rejuvenation.59,60
CONTRAINDICATIONS TO THE USE OF PN/PDRN
A patch test is warranted in patients who give a history of hypersensitivity to fish. Other contraindications to be mindful of are as mentioned [Table 4].
| 1. Age <18 years |
| 2. Pregnancy and lactation |
| 3. Active infection in the area of injection |
| 4. Autoimmune conditions |
| 5. Intolerance to the drug |
| 6. Systemic Infection |
| 7. Epilepsy |
PN: Polynucleotides, PDRN: Polydeoxyribonucleotides
CONCLUSION
PN/PDRN are biomaterials with proven efficacy in various regenerative and dermatological indications. This review presents developments that are pivotal in broadening the accessibility and acceptance of PDRN and PN-based therapies in various medical and esthetic fields. Although it highlights the strong regenerative potential of the biomaterials, more controlled studies are needed to bridge the gaps in the evidence-based use of these PNs. Thus, the present positioning of PN/PDRN use as a drug in the regenerative treatment segment is promising yet needs attention regarding certain critical issues.
Author contributions:
Gulhima Arora: Concepts, design, definition of intellectual content, literature search, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, manuscript review and guarantor.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
Patient’s consent is not required as patients identity is not disclosed or compromised.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Nucleic acids- nucleosides and nucleotides. 2022. Available from: https://microbenotes.com/nucleic-acids-nucleosides-and-nucleotides [Last accessed on 2025 Feb 12]
- [Google Scholar]
- Hyaluronate increases polynucleotides effect on human cultured fibroblasts. JCDSA. 2013;3:124-8.
- [CrossRef] [Google Scholar]
- Polydeoxyribonucleotides derived from salmon: Potential aesthetic applications and mechanisms of action. IOSR J Dent Med Sci. 2023;22:32-5.
- [Google Scholar]
- Recent advances on polydeoxyribonucleotide extraction and its novel application in cosmeceuticals. Int J Biol Macromol. 2024;282:137051.
- [CrossRef] [PubMed] [Google Scholar]
- From Polydeoxyribonucleotides (PDRNs) to polynucleotides (PNs): Bridging the gap between scientific definitions, molecular insights, and clinical applications of multifunctional biomolecules. Biomolecules. 2025;15:148.
- [CrossRef] [PubMed] [Google Scholar]
- From cosmetics to innovative cosmeceuticals-Non-woven tissues as new biodegradable carriers. Cosmetics. 2021;8:65.
- [CrossRef] [Google Scholar]
- Activation of adenosine A2A receptors restores the altered cell-cycle machinery during impaired wound healing in genetically diabetic mice. Surgery. 2011;149:253-61.
- [CrossRef] [PubMed] [Google Scholar]
- Skin boosters: Definitions and varied classifications. Skin Res Technol. 2024;30:e13627.
- [CrossRef] [PubMed] [Google Scholar]
- Biologic properties of polynucleotides. I. The anti-complementary activity of polynucleotides. J Clin Invest. 1963;42:1947-55.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological activity and clinical use of PDRN. Front Pharmacol. 2017;8:224.
- [CrossRef] [PubMed] [Google Scholar]
- Preparation of polydeoxyribonucleotide nanoliposomes and their applicability to cosmetic formulations. Curr Pharm Biotechnol 2025 Available from: https://www.eurekaselect.com/239771/article [Last accessed on 2025 Apr 16]
- [CrossRef] [Google Scholar]
- Polydeoxyribonucleotide: A promising skin anti-aging agent. Chin J Plast Reconstr Surg. 2022;4:187-93.
- [CrossRef] [Google Scholar]
- Analysis of skin regeneration and barrier-improvement efficacy of polydeoxyribonucleotide isolated from Panax ginseng (C.A. Mey.) adventitious root. Molecules. 2023;28:7240.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-inflammatory effect of polydeoxyribonucleotides (PDRN) extracted from red alga (Porphyra sp.) (Ps-PDRN) in RAW 264.7 macrophages stimulated with Escherichia coli lipopolysaccharides: A comparative study with commercial PDRN. Cell Biochem Funct. 2023;41:889-97.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effects of polydeoxyribonucleotides on cartilage degradation in experimental cultures. Cell Biochem Funct. 2013;31:214-27.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of polydeoxyribonucleotides on human fibroblasts in primary culture. Cell Biochem Funct. 1999;17:107-14.
- [CrossRef] [Google Scholar]
- Polydeoxyribonucleotide regulation of inflammation. Adv Wound Care. 2020;9:576-89.
- [CrossRef] [Google Scholar]
- Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev. 2020;59:101036.
- [CrossRef] [PubMed] [Google Scholar]
- Biostimulatory effects of polydioxanone, poly-D, L lactic acid, and polycaprolactone fillers in mouse model. J Cosmet Dermatol. 2019;18:1002-8.
- [CrossRef] [PubMed] [Google Scholar]
- Scar prevention and enhanced wound healing induced by polydeoxyribonucleotide in a rat incisional wound-healing model. Int J Mol Sci. 2017;18:1698.
- [CrossRef] [PubMed] [Google Scholar]
- An effective range of polydeoxyribonucleotides is critical for wound healing quality. Mol Med Report. 2018;18:5166-72.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro polydeoxyribonucleotide effects on human pre-adipocytes. Cell Prolif. 2008;41:739-54.
- [CrossRef] [PubMed] [Google Scholar]
- Polydeoxyribonucleotide promotes cyclobutane pyrimidine dimer repair in UVB-exposed dermal fibroblasts. Photodermatol Photoimmunol Photomed. 2007;23:242-9.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic effects of polydeoxyribonucleotide in an in vitro neuronal model of ischemia/reperfusion injury. Sci Rep. 2023;13:6004.
- [CrossRef] [PubMed] [Google Scholar]
- Polydeoxyribonucleotide-delivering therapeutic hydrogel for diabetic wound healing. Sci Rep. 2020;10:16811.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of topical polydeoxyribonucleotide on radiation-induced oral mucositis. Tech Innov Patient Support Radiat Oncol. 2018;7:17-9.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus report on the use of PN-HPT™ (polynucleotides highly purified technology) in aesthetic medicine. J Cosmet Dermatol. 2021;20:922-8.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging wound-healing injectable polydeoxyribonucleotide: Potential as a prohibited doping method and its simple detection via CRISPR/Cas12a system. Int J Biol Macromol. 2025;309:142999.
- [CrossRef] [PubMed] [Google Scholar]
- Points to ponder on the role of polynucleotides in regenerative and aesthetic medicine: A systematic review. Eur J Plast Surg. 2024;47:66.
- [CrossRef] [Google Scholar]
- Medical aesthetics-Current trends and a review of its applications. Indian Dermatol Online J. 2023;14:309-19.
- [CrossRef] [PubMed] [Google Scholar]
- Recognizing “medical aesthetics” in dermatology: The need of the hour. Indian J Dermatol Venereol Leprol. 2021;87:1-2.
- [CrossRef] [PubMed] [Google Scholar]
- Biorevitalization of the skin with skin boosters: Concepts, variables, and limitations. J Cosmet Dermatol. 2021;20:2458-62.
- [CrossRef] [PubMed] [Google Scholar]
- A survey on the cosmetic use of injectable polynucleotide: The pattern of practice among Korean Dermatologists. J Cosmet Dermatol. 2024;23:1243-52.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative evaluation of the effectiveness of novel hyaluronic acid-polynucleotide complex dermal filler. Sci Rep. 2020;10:5127.
- [CrossRef] [PubMed] [Google Scholar]
- Novel anti-melanogenesis properties of polydeoxyribonucleotide, a popular wound healing booster. Int J Mol Sci. 2016;17:1448.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic efficacy of 1,927-nm fractionated thulium laser energy and polydeoxyribonucleotide on pattern hair loss. Med Lasers. 2016;5:22-8.
- [CrossRef] [Google Scholar]
- Improvement of hair graying during a treatment of male pattern hair loss using 1,927-nm fractionated thulium laser energy and polydeoxyribonucleotide injections. Med Lasers. 2017;6:37-40.
- [CrossRef] [Google Scholar]
- Therapeutic efficacy of autologous platelet-rich plasma and polydeoxyribonucleotide on female pattern hair loss. Wound Repair Regen. 2015;23:30-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and tolerability assessment of a polynucleotide-based gel for improvement of pattern hair loss. Arch Dermatol Res. 2024;316:331.
- [CrossRef] [PubMed] [Google Scholar]
- Trophic effects of polynucleotides and hyaluronic acid in the healing of venous ulcers of the lower limbs: A clinical study. Int Wound J. 2016;13:754-8.
- [CrossRef] [PubMed] [Google Scholar]
- Polydeoxyribonucleotide for the improvement of a hypertrophic retracting scar-An interesting case report. J Cosmet Dermatol. 2020;19:2982-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of wound healing effects between Oncorhynchus ketaderived polydeoxyribonucleotide (PDRN) and Oncorhynchus mykissderived PDRN. Arch Craniofac Surg. 2018;19:20-34.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical evaluation of the trophic effect of polydeoxyribonucleotide (PDRN) in patients undergoing skin explants. A Pilot study. Curr Med Res Opin. 2001;17:128-31.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of polydeoxyribonucleotide on the treatment of radiating leg pain due to cystic mass lesion in inner aspect of right sciatic foramen: A CARE compliant case report. Medicine (Baltimore). 2018;97:e12794.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of polydeoxyribonucleotide injection in a patient with carpal tunnel syndrome. Am J Phys Med Rehabil. 2018;97:e93-5.
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant clinical effects of polydeoxyribonucleotide in lichen sclerosus. Eur J Dermatol. 2012;22:575-6.
- [CrossRef] [PubMed] [Google Scholar]
- PDRN, a bioactive natural compound, ameliorates imiquimod-induced psoriasis through NF-κB pathway inhibition and Wnt/β-catenin signaling modulation. IJMS. 2020;21:1215.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting adenosine receptor by polydeoxyribonucleotide: An effective therapeutic strategy to induce white-to-brown adipose differentiation and to curb obesity. Pharmaceuticals (Basel). 2021;14:728.
- [CrossRef] [PubMed] [Google Scholar]
- Polynucleotides HPT for Asian skin regeneration and rejuvenation. Clin Cosmet Investig Dermatol. 2024;17:417-31.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, patient/evaluator-blinded, split-face study to compare the efficacy and safety of polycaprolactone and polynucleotide fillers in the correction of crow's feet: The latest biostimulatory dermal filler for crow's feet. J Cosmet Dermatol. 2020;19:1593-9.
- [CrossRef] [PubMed] [Google Scholar]
- Preventive effect of polynucleotide on post-thyroidectomy scars: A randomized, double-blinded, controlled trial. Lasers Surg Med 2018
- [CrossRef] [PubMed] [Google Scholar]
- Polynucleotide-based treatments for various facial scars including combat injuries. J Dermatolog Treat. 2024;35:2426626. [Article in Press]
- [CrossRef] [PubMed] [Google Scholar]
- Preliminary prospective and randomized study of highly purified polynucleotide vs placebo in treatment of moderate to severe acne scars. Aesthet Surg J. 2021;41:P866-74.
- [CrossRef] [PubMed] [Google Scholar]
- Current practices and perceived effectiveness of polynucleotides for treatment of facial erythema by cosmetic physicians. Skin Res Technol. 2023;29:e13466.
- [CrossRef] [PubMed] [Google Scholar]
- Striae distensae and an innovative intradermal medical device based on PN HPTTM, hyaluronic acid, and mannitol. A real-world insight. J Case Rep Med Hist. 2024;4:1-4.
- [Google Scholar]
- Comparison of the effects of polynucleotide and hyaluronic acid fillers on periocular rejuvenation: A randomized, double-blind, split-face trial. J Dermatolog Treat. 2022;33:254-60.
- [CrossRef] [PubMed] [Google Scholar]
- Biorevitalization of postmenopausal labia majora, the polynucleotide/hyaluronic acid option. Obstet Gnecol Rep 2019 Available from: https://www.oatext.com/biorevitalization-of-postmenopausal-labia-majora-thepolynucleotide-hyaluronic-acid-option.php [Last accessed on 2025 Feb 28]
- [CrossRef] [Google Scholar]
- Poly-D,LLactic acid stimulates angiogenesis and collagen synthesis in aged animal skin. Int J Mol Sci. 2023;24:7986.
- [CrossRef] [PubMed] [Google Scholar]






