Translate this page into:
Role of Recipient-site Preparation Techniques and Post-operative Wound Dressing in the Surgical Management of Vitiligo
Address for correspondence: Dr. Iltefat Hamzavi, 3031 W Grand Boulevard, Suite 800, Detroit, Michigan - 48202, USA. Email: Ihamzav1@hfhs.org
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Vitiligo is an acquired skin disorder characterized by the destruction of melanocytes resulting in achromic macules and patches involving the affected skin. Multiple methods of treatments have emerged to manage vitiligo, including medical and surgical techniques. Among the surgical techniques described in the management of vitiligo are minipunch grafting, split-thickness skin grafting, hair follicle transplantation, suction blister grafting, and cultured and non-cultured autologous melanocyte transplantation. However, prior to grafting optimal recipient-site preparation is needed for graft survival and successful repigmentation outcomes. Similarly, post-operative care of the recipient site is vital to yielding a viable graft irrespective of the transplantation technique employed. This article reviews the multiple methods employed to prepare the recipient site in vitiligo surgeries and the post-surgical conditions which optimize graft viability. A pubmed search was conducted utilizing the key words listed below.
Keywords
CO2 laser
de-epithelialization
dermabrasion
epidermal ablation
Er:YAG laser
liquid nitrogen
PUVA
recipient-site
suction blister
vitiligo surgery
INTRODUCTION
Vitiligo is an acquired skin disorder characterized by the presence of depigmented macules and patches affecting 0.5-2% of the population.[1] Research investigations indicate that vitiligo is a multifactorial disease involving immunologic, environmental, and genetic components that ultimately result in melanocyte destruction.[2] Although vitiligo is physically asymptomatic, those affected often experience significant psychological distress.[3] This impact necessitates instigation of early and appropriate management of vitiligo in order to minimize disease progression and additional psychological tolls. For this purpose, numerous treatments have emerged including topical medications and photochemotherapy. However, in patients who fail to achieve repigmentation with conservative therapy, surgical interventions may be utilized.
The goal of surgical interventions in the management of vitiligo is to achieve cosmetically acceptable repigmentation of vitiliginous lesions. Although multiple surgical techniques have emerged, including minipunch grafting, split-thickness skin grafting, hair follicle transplantation, suction blister grafting, and cultured and non-cultured autologous melanocyte transplantation, the underlying principle for each techniques is the same; harvesting of autologous, active melanocytes from unaffected pigmented donor skin for transplantation to achromic recipient skin.[2] In order to achieve successful repigmentation, it is necessary to ascertain disease stability. The absence of new lesions, lack of progression of old lesions and lack of an isomorphic response within a period of about 1 year is sufficient to reflect disease stability.[4] Although the harvesting and transplantation techniques are important, the preparation and post-operative care of the recipient site is vital to yielding a viable graft irrespective to the transplantation technique employed.
ROLE OF RECIPIENT-SITE PREPARATION IN VITILIGO SURGERIES
Prior to any method of grafting, recipient-site preparation is required to allow access to the underlying structures necessary for melanocyte adherence and nutrition. To prepare the recipient site, the epidermis must be separated from the underlying dermis at approximately the dermo-epidermal junction where pinpoint bleeding is first seen. Removing this layer permits plasmatic imbibition for the first 24 to 48 hours and then vascular ingrowth to provide cellular nutrition thereafter.
Despite having many different techniques to prepare the recipient site, there are limited discussions comparing these techniques and their effect on repigmentation outcomes. It has been suggested that the preparation of the recipient site may be a critical step to achieving successful repigmentation as repigmentation of vitiliginous lesions at de-epithelialized areas by dermabrasion and suction blisters has been seen without subsequent grafting.[567] Thus, the techniques utilized in recipient-site preparation may be important in generating cosmetically acceptable repigmentation.
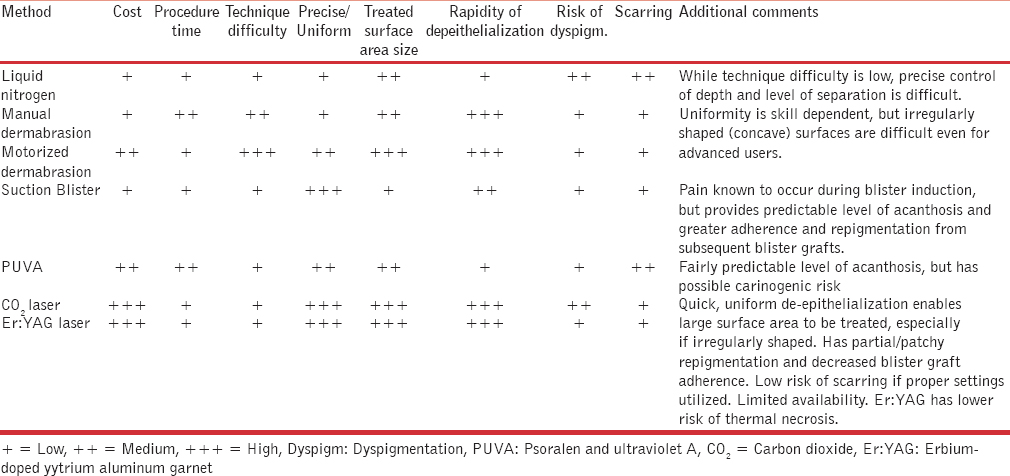
Multiple methods of recipient-site preparation have been described. These include the use of liquid nitrogen, mechanical dermabrasion, suction blisters, psoralen plus ultraviolet A (PUVA), and CO2 and Er:YAG lasers. The remainder of this section will discuss and compare the methods utilized in recipient-site preparation.
Liquid nitrogen
Liquid nitrogen is commonly used in cryosurgery as a method of selective tissue destruction through the rapid formation of intracellular and extracellular ice crystals. Subsequent thawing results in vascular stasis disrupting the microcirculation and creating greater amounts of tissue damage. This selective destruction is utilized in recipient-site preparation to induce blister formation.
Multiple studies have reported the use of liquid nitrogen in the preparation of vitiliginous lesions prior to grafting. Maleki et al. achieved blister formation through the administration of liquid nitrogen via a cotton swab applicator to unspecified anatomic locations with two, 15-20 second cycles and a 20 second thawing interval.[8] Similarly, Hann et al. reported blister formation at recipient sites located over the entire body, including the eyelid, genitals, hands, and back, within 24 hours after three to six freeze-thaw cycles with liquid nitrogen exposure times of three to five seconds per cycle.[9] In both studies, grafting onto recipient sites was performed with suction blisters obtained from donor sites. All patients achieved excellent repigmentation. However, cryotherapy is not without risk.
Recipient-site preparation with cryotherapy may be complicated by peripheral hypopigmentation, hyperpigmentation, and hypertrophic scarring.[9] Hypertrophic scarring and hyperpigmentation have been associated with excessive liquid nitrogen application resulting in severe inflammation. This adverse effect has primarily been reported in areas, such as the dorsum of the hands and fingers, where prolonged exposure to liquid nitrogen is required to induce blister formation.[9] With regards to perilesional dyspigmentation following cryotherapy, the adjuvant use of PUVA has been reported to reduce hypopigmented rims, and hyperpigmentation spontaneously resolves for most patients within 1 year.[9]
Dermabrasion
Recipient-site preparation with dermabrasion utilizes rapidly rotating abrasive tools (diamond fraise, wire brush, or serrated wheel) to remove the epidermis and superficial dermis. Of the various available abrasive heads, the diamond fraise generally creates a more uniform abrasion based upon histologic examinations.[10] Thus, dermabrasion for recipient-site preparation commonly involves the use of a high-speed dermabrader fitted with a diamond fraise steel wheel, set to 10,000 rpm, and applied to the vitiliginous site until pinpoint bleeding is achieved.[411]
Dermabrasion offers several advantages compared to other preparation techniques. One of the main advantages is that unlike all excisional procedures, including split or full-thickness grafting, scarring is a rare occurrence with dermabrasion.[12] In addition, the material cost to perform this procedure is low. Compared to blister-induction techniques, dermabrasion is a relatively quick method of recipient-site preparation. However, when compared to ablative laser techniques, less surface area can be prepared in a given timeframe.
Studies comparing laser preparation techniques to dermabrasion revealed equal to inferior efficacy of repigmentation with dermabrasion. In a study by Kahn et al. comparing the histological differences between dermabrasion and short-pulsed CO2 laser followed by split-thickness skin grafting, no significant difference in residual dermal tissue destruction was noted and grafting was equally effective for both methods.[13] A subsequent single patient comparison of recipient-site preparation with dermabrasion versus Er:YAG laser followed by transplantation of autologous cultured melanocytes demonstrated inferior repigmentation outcomes at the site prepared with dermabrasion compared to the Er:YAG laser. The significance of such findings is the efficacy of the autologous melanocyte transplantation may have been deemed either entirely successful or unsuccessful based upon the method utilized to prepare the achromic lesion.[11] Although no theory regarding the cause of varying outcomes was proposed, this finding further brings into question what role recipient-site preparation plays in repigmentation outcomes.
Despite findings that suggest equal or superior repigmentation outcomes with laser tissue ablation, the increased cost and availability of lasers poses a significant limitation to their use. Kaliyadan proposes a cheaper alternative using a ball-shaped radiofrequency ablating device at 50% maximum power in the cut/coagulation mode followed by light manual dermabrasion to achieve de-epithelialization.[14] This cheaper alternative provides the advantage of faster graft site preparation than normal dermabrasion. Furthermore, no complications of scarring were observed and no significant difference in the rate or degree of repigmentation was noted as compared to conventional dermabrasion.
In general, dermabrasion provides a quick and effective method of recipient-site preparation with minimal risks of scarring. It is both a more accessible and economic method of recipient-site preparation than lasers, but is highly dependent on the skill of the surgeon to achieve precise and uniform de-epithelialization. Ample experience and technical skills are essential to obtain consistent results, thus limiting the reproducibility of this technique compared to alternative methods of recipient-site preparation. The technique is also very difficult to do on concave surfaces such as peri-orbital lesions and soft tissues such as genitalia.[13]
Suction blisters
Suction blisters have been described as both a means to prepare the recipient site and technique for harvesting donor site tissue. This preparation method uses a negative pressure apparatus, such as a syringe, to generate a pressure of –300 to –500 mmHg and induce epidermal blister formation.[1315] The pressure required to induce epidermal blister formation depends upon the strength of the dermo-epidermal junction. Thus, lower pressure (-300 mmHg) is necessary to induce blister formation in elderly skin due to age-related weakening of the dermo-epidermal junction.
When compared to recipient-site preparation with CO2 laser, suction blisters appear to yield a more favourable microenvironment for subsequent melanocyte grafting. In Lee et al. comparison of repigmentation and suction blister graft adherence following these two preparation techniques, they observed superior outcomes in those whose recipient sites were prepared with suction blister.[1617] Unlike the permanent graft attachment with homogenous repigmentation noted in those with suction blisters preparation, epidermal grafts on sites prepared with the CO2 laser detached within 1-2 weeks and resulted in only partial repigmentation.[16] Similar findings were seen in a cultured, autologous melanocyte transplantation study comparing repigmentation outcomes of suction blisters versus CO2 laser recipient-site preparation.[18] The authors of this second study hypothesized this difference in repigmentation may be due to a more favourable microenvironment from adequate serous drainage in areas prepared by suction blisters, whereas areas prepared by the CO2 laser resulted in an unfavourable, dry and devitalized surface.[18] Thus, compared to CO2 laser, suction blister preparation of the recipient site appears to provide superior permanent graft adherence and a more cohesive, complete repigmentation.[13]
There are several limitations to this technique. Similar to liquid nitrogen, patients experience notable discomfort during the induction of blister formation. Secondly, preparation of vitiliginous lesions with suction blisters is a time-consuming task requiring between 15 minutes to more than three hours for blister induction. The exact time for blister induction depends upon the combination of diameter of the cup, anatomical location, age of the patient, amount of negative pressure, temperature, pathologic and individual variations, and addition of other manoeuvres, such as intraepidermal injection of saline or pre-treatment with topical psoralen plus ultraviolet A.[19] However, this preparation method can yield only limited amounts of skin making suction blisters an inferior method of preparation for large vitiliginous lesions, as compared to other methods.
Psoralen and ultraviolet A (PUVA)
The use of PUVA to de-epithelialize vitiliginous recipient sites is achieved through the induction of a phototoxic blister. Dermatologists have utilized this method for many years. Srivinas et al. describe a case report of a patient with stable, generalized vitiligo requiring preparation of a large recipient site.[20] For large recipient sites, the use of suction blisters, liquid nitrogen, or dermabrasion may be impractical. Thus, the use of PUVA in this patient permitted the timely preparation of an extensive vitiliginous area.
The method reported by Srivinas et al. involved the application of 0.075% 8-methoxypsoralen with exposure to 10 J/cm2 of UVA for two consecutive days prior to surgery in a PUVA full body unit 10 minutes after 8-MOP application.[19] Twenty-four hours later, the vitiliginous skin was erythematous, blistered, and ready for surgery. Vigorous rubbing of the affected skin with saline-soaked gauze subsequently removed the epidermis. Any residual epidermis was then removed by dermabrasion with a wire brush to yield a large recipient site ready for melanocyte grafting.
In patients with large vitiliginous lesions such as the one described, the use of PUVA to induce a phototoxic blister is a time-efficient method of recipient-site preparation for the physician. Moreover, no scarring of the recipient site should occur since the phototoxic blisters spare the reticular dermis. However, there is concern for carcinogenesis, particularly in fairer skin types 1 and 2.[21]
Carbon dioxide (CO2) laser
The CO2 laser is a common method of tissue ablation in dermatology. The emission wavelength, 10,600 nm, is in the infrared portion of the electromagnetic spectrum and strongly absorbed by water. Targeting this chromophore enables rapid heating and vaporization of intracellular water to cause tissue destruction.[22] CO2 lasers were originally utilized as surgical cutting tools, emitting continuous wave beams. To selectively remove the epidermis and superficial dermis without involving deeper tissue, the distance between the skin and the laser head can be increased. This separation allows the CO2 beam to defocus and vaporize the tissue without cutting or coagulation, but does not prevent unsightly scarring from extensive thermal necrosis by continuous CO2 lasers. Therefore, all surgeons prefer the use of intermittent beams from pulsed or Ultrapulse lasers to reduce thermal damage and thereby prevent scarring. Additionally, scanning devices have been developed to increase the reproducibility and speed of epidermal ablation. For recipient-site preparation, CO2 laser ablation provides the benefits of rapid preparation with easy depth control. This depth control is particularly important on delicate and irregularly shaped skin, such as the mouth, eyes, and nose where precise de-epithelialization is key.[23]
Park et al. described the use of a conventional CO2 laser attached to a Silktouch Flashscanner to prepare the recipient site for epidermal grafting. Depending upon the location of the lesions, one to two passes of the laser at 1.0-3.0 W were applied, with subsequent removal of tissue debris using saline-soaked cotton tips.[24] With this method, magnification revealed near-complete de-epithelialization without evidence of bleeding, which would indicate either thermal coagulation or minimal injury to the underlying dermis. Ko et al. describe a similar method using a Silktouch Flashscanner attached to a CO2 laser (4.5 W, 0.2 sec pulse duration, 0.4 sec rest duration, 3-mm laser spot size) followed by epidermal blister grafting.[23] Excellent repigmentation was achieved in 80% of patients. Hyperpigmentation was noted as a complication in some patients. However, the hyperpigmentation gradually lightened and good color match was observed 6 months postoperatively.
An alternative method of CO2 laser recipient-site preparation has been described involving a Short-pulsed CO2 laser and Ultrapulse CO2 laser equipped with a computerized pattern generator (CPG).[525] The CPG is an automatic scanning device which allows rapid and precise tissue ablation.[5] In both cases, one pass of the laser was sufficient to remove the epithelium. Subsequent epidermal grafting yielded excellent repigmentation in the majority of patients six months postoperatively.
The benefit of using a Short-pulsed or Ultrapulse CO2 laser ablation for recipient-site preparation is the rapid, uniform, and reproducible de-epithelialization achieved with a single laser pass yielding minimal thermal damage; whereas the use of a conventional CO2 lasers has a greater risk of uneven epidermal ablation resulting in scarring and hyperpigmentation. Additional scanning equipment, such as CPG and the Silktouch Flashscanner, provide more precise and uniform tissue ablation with increased reproducibility and decreased operator variability. Moreover, histological evaluation demonstrated precise depth control and uniformity of tissue ablation without evidence of tissue necrosis when appropriate settings are applied.[1326]
Another method for epithelial ablation is fractional CO2 lasers, which result in the ablation of a column or “fraction” of tissue leaving the remaining, intervening skin untreated. The untreated tissue yields more rapid re-epithelializaion and may minimize the adverse effects of post-procedural erythema, oozing and crusting as well as post-inflammatory hyperpigmentation which may be observed with other forms of laser tissue ablation.[27] Although no studies have been published describing the role of fractional CO2 lasers in vitiligo surgeries, Passeron reports that the use of fractional CO2 lasers and micro-needles results in adequate penetration and adherence of melanocytes depicting interest in utilizing such methods clinically for recipient-site preparation.[28]
Complications of CO2 laser ablation include transient hyperpigmentation and uneven repigmentation, but the risk of permanent scarring from Short-pulsed and Ultrapulse CO2 lasers is very low. However, laser ablation adds significant financial costs to the procedure. Thus, the speed, uniformity and precision of the CO2 laser denote its utility in recipient-site preparation, most notably for large, irregular, or anatomically delicate lesions with the drawbacks of increased costs, uneven repigmentation and transient hyperpigmentation.
Erbium-doped yttrium aluminium garnet (Er:YAG) laser
The Er:YAG laser is another laser utilized in recipient-site preparation. This laser also achieves selective vaporization of water in superficial cutaneous tissue, but through the emission of a 2940 mm wavelength, which water molecules absorb 12-18 times more efficiently than the 10,600 nm wavelength of the CO2 laser. Moreover, its depth of penetration is only one-sixth that of CO2 lasers, and thus allows more effective and precise tissue ablation without associated thermal necrosis.[21]
Multiple authors described the use of the Er:YAG laser in recipient-site preparation. Yang et al. reported epidermal ablation was achieved following four to six passes of the pulsed Er:YAG laser (2 mm spot size, 500 mJ/pulse, 3.5 W, frequency 7 Hz) prior to suction blister grafting.[29] Results revealed 84% of patients achieved complete repigmentation one month postoperatively. In one-third of patients, temporary hyperpigmentation was noted at the grafted site, but no other complications were noted at either the recipient or donor site. Another study reported pinpoint bleeding of the recipient site was achieved with three to five passes of the pulsed Er:YAG laser (spot size 1.6-3 mm, 300-450 mJ/pulse, frequency 5 Hz) prior to transplantation of cultured melanocytes. This combination of recipient-site preparation and transplantation techniques yielded partial to complete repigmentation in 55% of treated lesions.[30] Of note, no anaesthesia was required for this method of recipient site tissue ablation.
In addition, the Er:YAG laser is noted to be a proficient method of recipient-site preparation removing 50 cm2 of the epidermis in seven to ten minutes.[31] This proficiency is particularly efficacious in cases of large leukoderma, such as those seen in piebaldism. Extensive de-epithelialization with the Er:YAG laser was described by Guerra et al. in the preparation of piebaldism recipient sites with one pass of the laser (spot size 2mm, 200-500 mJ, 6.37-15.92 J/cm2 fluence).[31] Histological examination confirmed complete de-epithelialization with minimal thermal injury of the papillary dermis. Similar to Kaufmann et al. study, most of their patients did not require anaesthesia for recipient site tissue ablation.[3031]
Moreover, Mulekar SV and Al Issa A, compared the use of laser and motorized dermabrasion to prepare one vitiliginous lesion on the foot on two patients with subsequent histological examination. They found that laser ablation has the increased precision and ease of operator-use as well as a bloodless field generated with the use of the Er:YAG laser. Histopathology revealed a more superficial ablation, more neutrophilic infiltrate and no collagen re-modelling with dermabrasion, while laser showed deeper abrasion, more lymphocytic infiltration and collagen re-modelling with clubbing of rete ridges observed at 90 days postoperatively. However, pigmentation outcomes and healing were similar in both sites (A. Al Issa, MD, S.V. Mulekar, MD, oral and email communication, September 18, 2014).
In summary, the benefits of utilizing the Er:YAG laser for recipient-site preparation include efficient and controlled tissue ablation with minimal risks of thermal injury and scarring. Similar to the CO2 laser, the Er:YAG laser is useful in preparing large, irregular, or delicate lesions. Moreover, there is minimal pain associated with use of the Er:YAG laser eliminating the need for anaesthesia. Such advantages make it an effective and valuable tool in the preparation of recipient sites.
ROLE OF POST-SURGICAL DRESSINGS IN VITILIGO SURGERIES
Adherence and survival of transplanted melanocytes relies upon the same principles required for basic wound healing: Minimizing the infectious bacterial load, local mechanical, phototoxic and chemical trauma to cells, and graft nutritional deficiencies. By optimizing these conditions, cell viability is maximized to promote graft survival and faster wound healing while decreasing the risk of dyspigmentation and scarring. The application of these principles in vitiligo surgeries will be discussed below in the text. A summary of the dressing class’ characteristics and indications can be found in Table 1.[3233]
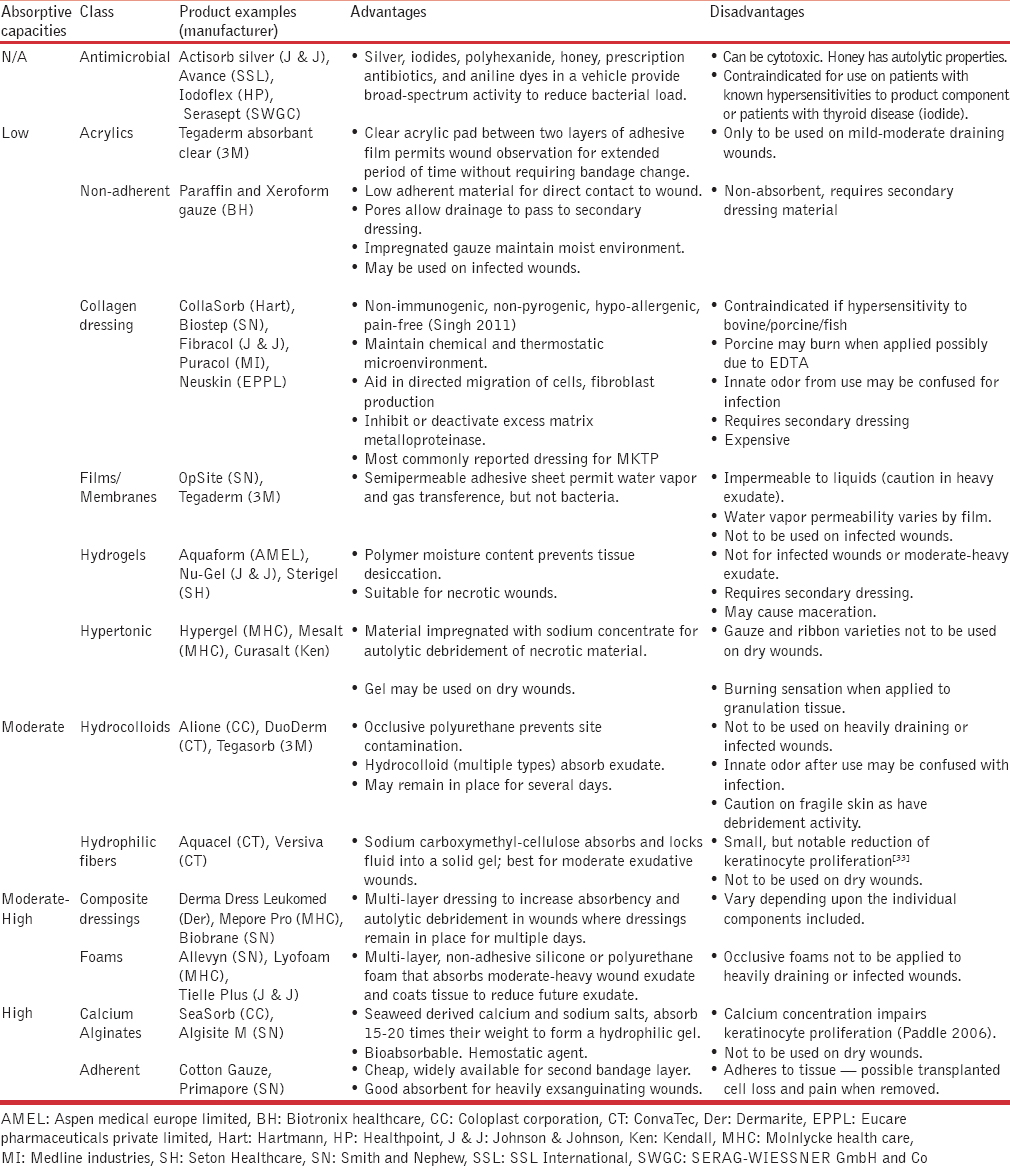
Bacterial load
The infectious bioburden in wounds does not become an issue until the bacterial load exceeds 105 colony-forming units per gram of tissue.[34] As all vitiligo surgical procedures employ pre-operative cleansing with antiseptic solutions and application of aseptic techniques, the risk of operative-related bacterial contamination is greatly reduced. Post-operative bandaging is frequently employed, if feasible, for multiple advantageous healing properties, one of which is the prevention of bacterial contamination.
To prevent post-operative contamination of wounds healing by secondary intent, wound dressings should be utilized. However, antiseptic solutions and direct wound contact with gauze (dry or moist) are to be avoided primarily due to resulting chemical and mechanical trauma, respectively;[35] these injuries are discussed below in greater detail. Due to the short time before re-epithelialization, low risk of bacterial load, and increased risk of contact dermatitis, antibiotic dressings or ointments are not encouraged in vitiligo surgical dressings.
It should be noted that green discoloration of the bandage does not necessarily indicate a clinical infection, such as pseudomonas. In the authors’ experience, these patients do not have subjective or objective findings consistent with an overt wound infection, a delay in re-epithelialization, nor a positive wound/dressing culture consistent with organisms producing green pigmentation. Rather, it appears myeloperoxidase may be the cause of this discoloration. This enzyme was originally called verdeperoxidase as it strongly absorbs 680 nm creating a green coloration compared to other heme-containing peroxidases.[3637] Pulmonary investigations have shown that green sputum both correlates with myeloperoxidase levels and persist following chemical extraction of green compounds, including pyocyanin and fluorescin.[3839] Thus, green discoloration of surgical dressings without concurrent infectious findings is likely due to myeloperoxidase rather infectious etiologies.
Chemical, mechanical, and phototoxic injuries
Chemical and photoinduced trauma, such as exposure to cytotoxic chemicals and ultraviolet light, may greatly impact the survival of newly transferred melanocytes and keratinocytes. While antiseptic solutions are frequently used to reduce wound bacterial load and thus accelerate healing, they often are found to be toxic to in-vitro host cells.[40] The degree of in-vivo cytotoxicity remains a highly debated topic in wound healing literature. Furthermore, antibiotic ointments and creams are common causes of contact dermatitis. Their activation of inflammatory pathways can lead to cell death via immune-mediated chemical insults. Therefore, given the low risk of vitiligo surgical infection and increased risk of transplanted cell death though direct cytotoxicity or indirect inflammatory responses, the use of antiseptic and antibiotic compounds is not recommended nor commonly employed for these surgical wounds. Additionally, caution must be exercised when re-exposing recipient sites to natural or artificial ultraviolet light, as newly epithelialized skin may be more sensitive than the pre-operative vitiliginous areas in the author's experience.
Sheer forces and the removal of adherent bandages are the primary causes of mechanical trauma in regards to melanocyte grafts. Therefore, one of the post-operative bandaging and care goals is to prevent transplanted cell removal, especially during the initial 72 hours. During this timeframe, the cells “take” to the exposed dermis and induce an ingrowth of capillaries with subsequent angiogenesis to maintain permanent graft viability. Thus, patients are commonly instructed not to expose bandages to water (showering and bathing) in order to prevent early dressing removal and the “washing off” of non-adhered transplanted cells. Since wound bed dehydration increases the risk of adherence to the bandaging and cellular desiccation, the use of occlusive films or hydrocolloids, such as Tegaderm and DuoDERM respectively, plus avoidance of direct wound contact with gauze is advised. On the other hand, excess wound hydration may lead to tissue maceration and possible increased risk of infection. Therefore, the authors of this manuscript frequently utilize a gauze tail, which extends beyond the occlusive dressing, to permit drainage of excess serous fluid. Once re-epithelialization is complete, gauze and other non-occlusive coverings may be applied to prevent traumatic removal of this partially adherent layer. Continued protection from physical trauma may be necessary up to two weeks following the operation, particularly for recipient sites prepared by laser ablation in blister graft transplantation, as previously discussed.[16]
Graft nutrition
Unlike normal skin, the nutritional source for transplanted cells varies depending upon the time since surgery. Initially, nutrition is delivered by diffusion of serous drainage through a process called plasmatic imbibition. After the first 48 to 72 hours, serous drainage generally begins to decrease and nutrition is primarily attained from capillary ingrowths during inosculation. As the graft continues maturing following day 5, angiogenesis ensures adequate nutrition to the newly developing tissue.
The presence and/or type of post-operative dressing employed may affect each of these stages. Prior to capillary ingrowth and re-epithelialization, excess absorbent dressing or lack of occlusion may impair adequate cellular hydration and nutrition. Once capillary ingrowth is initiated, traumatic relocation of transplanted cells may impair graft survival by potentially decreasing the cells’ proximity to vessels when the serous drainage subsides. Prior to obtaining adequate vasculature ingrowth for the cell's nutritional and oxygen demands, wound occlusion may cause a relative increase in tissue hypoxia and hypercapnic-induced acidic pH, thereby prompting further angiogenesis via an increased production and binding of keratinocyte-derived vascular endothelial growth factor, VEGF.[4142] Thus, the choice of dressing materials utilized in the immediate post-operative period may significantly effect graft survival and subsequent repigmentation.
SUMMARY
Prior to any vitiligo surgical graft, it is necessary to de-epithelialize the recipient skin for melanocyte adherence and survival. Several methods of recipient-site preparation exist including the use of liquid nitrogen, mechanical dermabrasion, suction blisters, PUVA, plus CO2 and Er:YAG lasers.Table 2 summarizes the advantages and disadvantages of each method of recipient-site preparation. Based upon operator preference, advantage profiles, and scarcity of published side-by-side technique comparisons, mechanical dermabrasion, suction blisters, and thermal ablation have become the predominant methods of de-epithelialization reported in the literature. Future controlled studies need to be performed to establish an evidence-based choice of recipient-site preparation in vitiligo surgery.
In addition to preparation techniques, the choice of multiple dressing materials to minimize any chemical, mechanical, or phototoxic traumas and enhance cell survival after the procedure is vital to optimizing graft repigmentation outcomes. This is particularly true during the first 48 to 72 hours when transplanted cells are initially attempting to incorporate themselves into the recipient site's tissue. However, protection from trauma, especially physical, may remain important for at least two weeks post-operatively depending upon the combination of recipient-site preparation and grafting technique. Despite a lack of evidence for the optimal dressing composition, collagen dressings have become the most commonly reported in cell suspension procedures as they theoretically could improve cell survival/migration. Dressings containing antimicrobials should be used with caution due to cytotoxicity, and hydrophilic fibers and calcium alginate dressings should be avoided due to innate keratinocyte impairment. While the majority of literature focuses the transplantation technique, recipient-site preparation and post-surgical dressings may significantly impact graft survival and thereby affect repigmentation outcomes. Thus, prospective comparison trials are necessary to identify the optimal recipient-site preparation technique and cost-effective dressing.
Source of Support: Nil.
Conflict of Interest: Dr. Al-Hadidi, Dr. Griffith and Dr. Aljamal has no conflicts of interest to disclose. Dr. Hamzavi is an investigator for Clinuvel, Estee Lauder, and Ferndale Laboratories. He receives grant money from these investigations and has no additional conflicts of interest to disclose.
REFERENCES
- Vitiligo surgery. In: Robinson JK, ed. Surgery of the Skin: Procedural Dermatology (2nd ed). UK: Elsevier Health Sciences; 2010. p. :683-92.
- [Google Scholar]
- The effectiveness of therapeutic interventions on quality of life for vitiligo patients: A systematic review. Int J Nurs Pract. 2012;18:396-405.
- [Google Scholar]
- Tissue grafts in vitiligo surgery — past, present, and future. Indian J Dermatol. 2009;54:150-8.
- [Google Scholar]
- Suction blister grafting with CO(2) laser resurfacing of the graft recipient site for vitiligo. J Dermatol. 2007;34:490-2.
- [Google Scholar]
- Comparison of two surgical approaches for treating vitiligo: A preliminary study. Int J Dermatol. 2002;41:135-8.
- [Google Scholar]
- Dermabrasion may repigment vitiligo through stimulation of melanocyte precursors and elimination of hyperkeratosis. J Cosmet Dermatol. 2012;11:318-22.
- [Google Scholar]
- Treatment of vitiligo with blister grafting technique. Iranian J Dermatol. 2008;11:55-9.
- [Google Scholar]
- Treatment of stable vitiligo with autologous epidermal grafting and PUVA. J Am Acad Dermatol. 1995;32:943-8.
- [Google Scholar]
- Preparation of Recipient Vitiliginous/Leukodermic Areas. In: Gupta DK, ed. Microskin Grafting for Vitiligo (1st ed). London: Springer; 2009. p. :41-3.
- [Google Scholar]
- Autologous cultured melanocytes in vitiligo treatment comparison of two techniques to prepare the recipient site: Erbium-doped yttrium aluminum garnet laser versus dermabrasion. Dermatol Surg. 2012;38:809-12.
- [Google Scholar]
- Vitiligo: Repigmentation with dermabrasion and thin split-thickness skin graft. Dermatol Surg. 1995;21:295-300.
- [Google Scholar]
- Grafting following short-pulse carbon dioxide laser de-epithelialization. Dermatol Surg. 1996;22:965-8.
- [Google Scholar]
- Using the ball-shaped attachment of a radiofrequency ablation device for preparation of recipient site in vitiligo surgery. Indian J Dermatol. 2011;56:459-60.
- [Google Scholar]
- Comparison of recipient site preparations in epidermal grafting for vitiligo: Suction blister and CO2 laser. J Eur Acad Dermatol Venereol. 2009;23:1448-9.
- [Google Scholar]
- The use of suction blisters for recipient site in epidermal grafting: The implications for vitiligo. J Eur Acad Dermatol Venereol. 2009;23:241-2.
- [Google Scholar]
- De-epithelialization of vitiliginous area for transplantation of cultured autologous melanocyte: A case report of two patients with different methods. Int J Dermatol. 2012;51:747-9.
- [Google Scholar]
- Suction blister epidermal grafting. In: Gupta S, Olsson MJ, Kanwar A, Ortonne JP, eds. Surgical Management of Vitiligo. Oxford: Blackwell Publishing; 2007. p. :96-107.
- [Google Scholar]
- Meshed split skin graft for extensive vitiligo. Indian J Dermatol Venereol Leprol. 2004;70:165-7.
- [Google Scholar]
- PUVA Follow-Up Study. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: A 30-year prospective study. J Am Acad Dermatol. 2012;66:553-62.
- [Google Scholar]
- Suction blister epidermal grafts combined with CO2 laser superficial ablation as a good method for treating small-sized vitiligo. Dermatol Surg. 2009;35:601-6.
- [Google Scholar]
- Recipient site preparation for epidermal grafting using carbon dioxide Silktouch laser. Clin Exp Dermatol. 2010;35:443-4.
- [Google Scholar]
- Treatment of vitiligo with suction epidermal grafting by the use of an ultrapulse CO2 laser with a computerized pattern generator. Dermatol Surg. 2001;27:565-8.
- [Google Scholar]
- Histological and clinical evaluation of facial resurfacing using a carbon dioxide laser with the computer pattern generator. Arch Otolaryngol Head Neck Surg. 1997;123:929-34.
- [Google Scholar]
- Fractional CO2 laser resurfacing as monotherapy in the treatment of atrophic facial acne scars. J Cutan Aesthet Surg. 2014;7:87-92.
- [Google Scholar]
- Micro Holes for Delivering Melanocytes in Vitiligo Skin. An Ex Vivo Approach as Compared to Laser-Assisted Dermabrasion. International Pigment Cell Conference. Lecture Conducted from Shangri-La, Singapore. 2014
- [Google Scholar]
- Treatment of vitiligo with autologous epidermal grafting by means of pulsed erbium: YAG laser. J Am Acad Dermatol. 1998;38:280-2.
- [Google Scholar]
- Grafting of in vitro cultured melanocytes onto laser-ablated lesions in vitiligo. Acta Derm Venereol. 1998;78:136-8.
- [Google Scholar]
- Permanent repigmentation of piebaldism by erbium: YAG laser and autologous cultured epidermis. Br J Dermatol. 2004;150:715-21.
- [Google Scholar]
- Collagen dressing versus conventional dressings in burn and chronic wounds: A retrospective study. J Cutan Aesthet Surg. 2011;4:12-6.
- [Google Scholar]
- Effect of different wound dressings on cell viability and proliferation. Plast Reconstr Surg. 2006;117(Suppl):110S-20S.
- [Google Scholar]
- Assessing the bioburden in poorly healing wounds. Wounds International. 2011;2:17-9.
- [Google Scholar]
- National Collaborating Centre for Women’ and Children’ Health (UK). Surgical Site Infection: Prevention and Treatment of Surgical Site Infection. 2008. London: RCOG Press; (NICE Clinical Guidelines, No.74.) Available from: http://www.ncbi.nlm.nih.gov/books/NBK53731/
- [Google Scholar]
- Structure of the green heme in myeloperoxidase. Arch Biochem Biophys. 1995;316:653-6.
- [Google Scholar]
- Assessment of airway neutrophils by sputum colour: Correlation with airways inflammation. Thorax. 2001;56:366-72.
- [Google Scholar]
- Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61:1281-7.
- [Google Scholar]
- Local environment of chronic wounds under synthetic dressings. Arch Dermatol. 1986;122:52-7.
- [Google Scholar]
- pH regulates vascular endothelial growth factor binding to fibronectin: A mechanism for control of extracellular matrix storage and release. J Biol Chem. 2004;279:2307-15.
- [Google Scholar]






