Translate this page into:
Spontaneous and induced degradation of dermal fillers: A review
*Corresponding author: Uwe Wollina, Department of Dermatology and Allergology, Municipal Hospital Dresden, Friedrichstrasse 41, Dresden, Germany. uwollina@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Wollina U, Goldman A. Spontaneous and induced degradation of dermal fillers: A review. J Cutan Aesthet Surg. 2024;17:273-81. doi: 10.4103/JCAS.JCAS_137_23
Abstract
Dermal fillers are among the most versatile tools in esthetic medicine. A broad range of temporary, semipermanent, and permanent filler products are on the market. We performed a narrative review on spontaneous and induced degradation of dermal fillers in vitro and in vivo. Hyaluronic acid-based fillers are the most frequently used temporary fillers. The products differ in their hyaluronic acid content, cross-linking, and rheological parameters. Endogenous hyaluronidase and reactive oxygen species are responsible for the spontaneous degradation of these fillers. Hyaluronidase digests the filler material by cleavage of the β-1,4 glycosidic linkage between N-acetylglucosamine and D-glucuronic acid. The enzyme can be used for treatment of medical and cosmetic adverse events due to hyaluronic acid-based filler. Higher hyaluronidase content and higher degree of cross-linking are major factors contributing to filler persistence over time. Semipermanent fillers are poly-(D), l-lactic acid and calcium hydroxylapatite. These filler types are decomposed by hydrolysis and osteoclastic enzymes. They usually persist up to 2 years, in single patients even more than 5 years. Sodium thiosulfate can stimulate degradation of calcium hydroxylapatite, but it is slow acting and not effective in case of emergency. Permanent fillers may show some kind of modification in situ, but spontaneous or induced degradation has not been documented. Once implanted the permanent fillers remain lifelong. Intralesional laser treatment supports the removal of permanent filler material as an alternative to surgery. Besides biocompatibility and toxicity, filler materials should also be assessed for degradation to improve patient safety.
Keywords
Calcium hydroxylapatite
Degradation
Dermal filler
Hyaluronic acid
Polyalkylimide
Polylactic acid
Polymethacrylate
INTRODUCTION
Esthetic procedures have gained an increasing interest in the last decades. Patients prefer more and more less invasive techniques with a short if any downtime. Therefore, filler injections have become one of the most frequently used approaches to combat signs of aging, restore volume, and sculpt the face.1
All fillers are polymeric biomaterials, that is macromolecules consisting of many monomers, in linear, branched or cross-linked 3D-structures. Dermal fillers can be classified into three major types: temporary, semipermanent, and permanent [Table 1].2-4 Temporary and semipermanent fillers are subject to biodegradation. This is a process that results in a gradual breakdown of the injected material by specific biological activity. Degradation of dermal fillers occurs through either surface or bulk erosion or a combination of both.
| Type | Examples |
|---|---|
| Temporary | Hyaluronic acid, efficacy for a limited time mostly 1 year, eventually the material becomes completely decomposed |
| Semipermanent | Poly-(d,) l-lactic acid and calcium hydroxylapatite, efficacy usually up to 2 years, biostimulatory (collagen), material completely resorbable |
| Permanent | Polymethylmethacrylate and liquid silicon, efficacy for years but limited by possible filler migration and late adverse events, nondegradable |
In contrast, permanent fillers prepare scaffolds with varying porosity, geometry, surface area, and biophysical characteristics. The material injected does not undergo any biodegradation but may be modified otherwise.5
Degradation of hyaluronic acid fillers (HAFs) is mediated by hyaluronidase and free radical oxidation reactive oxygen species (ROS). Hyaluronic acid (HA) is composed of glucuronic acid and N-acetyl-D-glucosamine linked by β(1,4)-glycosidic bond. The hyaluronidase enzymes are endo-N-acetylhexosaminidases that digest HA polymers through enzymatic cleavage of the β-1,4 glycosidic linkage between N-acetyl-glucosamine and D-glucuronic acid. In humans, six different hyaluronidases have been identified.6,7
In a woman undergoing abdominoplasty, abdominal skin was superficially injected with 0.1–0.2 mL of 5 different HAFs. Immediately after filler injection, 4 IU of five different hyaluronidase products were applied into the filler deposits and repeated every 2 min until the swelling was gone. Histological analysis was done on the surgical specimen. It was demonstrated that the five hyaluronidase products displayed a wide range of doses and times to completely degrade the HAFs ranging from less than 2 min to more than 16 min.8
In another trial, the role of hyaluronidase amount was investigated in 15 human participants. Seven commercially available HAFs were used. Fillers were injected on the back followed by either 20 IU or 40 IU hyaluronidase. Follow-up was 14 days. There was a significant degradation of all HAFs by hyaluronidase, but surprisingly no significant difference between the two dosages used.9 These results are controversial since other authors came to contrary conclusions. In one trial with human participants, a correlation between the hyaluronidase dose and HAF degradation was noted. The onset of the degradation was fast for all HAFs.10
Degradation in vivo is an important factor limiting durability of desired filler effects. The resulting byproducts may bear a potential risk of adverse events. Intended degradation is important in case of cosmetic or medical adverse events, to remove the filler material, if possible. The kinetics of this process are crucial for unintended vascular compromise by filler injection.
In this narrative review, we performed a literature search on spontaneous and induced degradation of dermal fillers on Pubmed. In vitro and in vivo studies (animal and human) were considered. We will focus on the major filler types and present data on spontaneous and induced degradation.
HYALURONIC ACID FILLERS
HAFs are the most versatile fillers, and their injection is among the most commonly performed aesthetic techniques. HAFs are developed using HA powder and a cross-linker to prevent rapid enzymatic degradation and increase durability. There is a wide spectrum of products with different cohesive and viscoelastic properties on the market. Key qualities of HAFs are HA content, type, and degree of cross-linking, and washing processes. The products also may contain a local anesthetic like lidocaine.3,11
HAF can be divided into mono- and biphasic fillers. Biphasic fillers contain a mixture of cross-linked HA and a carrier of non cross-linked HA. Monophasic HA fillers are produced through cross-linking by varying the percentage of high- and low-molecular weight HA.12
Despite the fact that HAFs are temporary fillers, clinical experience argues for extensive longevity of filler deposits injected in the subcutaneous fat of the lateral face, midfacial deep fat compartments, and chin. Magnetic resonance imaging (MRI) investigations demonstrated persistence of HAF up to 27 months in the lateral face and mid-face in single patients.13
A trial investigated the spontaneous degradation of the HAFs 99 fill, Juvederm Voluma with Lidocaine, Neuramis Volume Lidocaine, Restylane Lyft with Lidocaine, and Yvoire Contour plus in hairless mice. The injection volume was 0.1 mL. The volume of the fillers increased in situ for up to 2 months and gradually decreased from 2 months to 18 months. The authors established a final kinetic model based on a one-compartment degradation. The swelling of HAF follows first-order kinetics in vitro in accordance with the following equation: dQtdt=k (Qe-Qt). Q indicates the swelling ratio, W0 describes the dehydrated weight of the HAF, while Wt stands for the hydrated weight at the time point t. The swelling ratio of the equilibrium of the HAF is Qe, Qt represents the swelling ratio at the time point t. The proportionality constant between the rate of swelling and the unrealized swelling capacity is k. The complete decomposition time for the investigated commercially available HAFs varied between 740 days for the biphasic Restylane Lyft with Lidocaine to 2050 days for Yvoire Contour plus.14
Another in vitro study investigated eight different commercially available HAFs with a HA content between 8.7 and 27 mg/mL. Of each HAF, 4 mg/mL in phosphate buffered saline (PBS) were incubated in with 5 IU/mL or 50 IU/mL bovine testis hyaluronidase (Sigma–Aldrich, Milan, Italy) at 37°C under stirring condition. The amount of soluble HA was quantified by the carbazole assay. Eventually, the different HAFs were classified as low to very high stability gels [Table 2].15
| Class | Time to complete | Examples | HA (mg/mL) degradation |
|---|---|---|---|
| Light stability | 15 h, 5 IU BTH | Restylane by Q-MED | 20 |
| Medium stability | 3–24 h, 50 IU BTH | Amalian I by S&V Technologies | 8.7 |
| Viscofill Basic by Gelfipharma Int. | 12 | ||
| 10 days, 50 IU BTH, | Amalian II by S&V Technologies | 24 | |
| 80% degradation | Amalian III by S&V Technologies | 24 | |
| Amalian Lips by S&V Technologies | 24 | ||
| 10 days, 50 IU BTH, | Viscofill Medium by Gefipharma Int. | 18 | |
| <40% degradation | Viscofill Extra by Gelfipharma Int. | 27 | |
| High stability | 10 days, 50 IU BTH, | Amalian II® by S&VTechnologies | 24 |
| 80% degradation | Amalian III® by S&V Technologies | 24 | |
| Amalian Lips® by S&V Technologies | 24 | ||
| Very high stability | 10 days, 50 IU BTH, | Viscofill Medium® by Gefipharma Int. | 8.7 |
| < 40% degradation | Viscofill Extra® by Gelfipharma Int. | 18 | |
| 10 days, 50 IU BTH, | Amalian II by S&V Technologies | 27 |
HAF: Hyaluronic acid fillers, BTH: Bovine testicular hyaluronidase, HA: Hyaluronic acid
The efficacy of hyaluronidase (Hylenex—a recombinant hyaluronidase from Halozyme Therapeutics Inc., San Diego, CA, US) to decompose HAFs was evaluated in vitro. Restylane, Juvederm Voluma, and Belotero Balance were exposed to 450 IU hyaluronidase in vitro for 5 and 30 min. A laser-based particle size analyzer revealed only modest changes in particle size [Table 3].16
| Filler | Particle size in µm untreated | Particle size in µm after 30 min with hyaluronidase |
|---|---|---|
| Restylane | 472 | 419 |
| Juvederm Voluma | 703 | 635 |
| Belotero Balance | 410 | 345 |
HAF: Hyaluronic acid filler
Three commercially available HAFs (Belotero Balance with Lidocaine, Emervel classic with Lidocaine, and Juvederm Ultra 3 with Lidocaine) were labeled with a fluorescent linker dye (PKH67, Sigma) and exposed to 10 IU of bovine testicular hyaluronidase (Hylase “Dessau”, Riemser Pharma, Greifswald, Germany). During HAF degradation by hyaluronidase the dye became released (decreased fluorescence intensity). The process was documented by time-lapse video-microscopy over 20 h. HAF degradation was measured as difference in fluorescence of HAF plus hyaluronidase versus HAF with sodium chloride (NaCl). Degradation of Belotero Balance with Lidocaine started at 5 h and reached significance from 14 h onwards. Degradation of Emervel classic with Lidocaine began at 7 h and reached significance from 13 h onwards. There was no significant degradation observed for Juvederm Ultra 3 with Lidocaine with the 10 IU Hylase “Dessau”, while higher dosages might have worked better.7
The same group has used time-relapsed video-microscopy for degradation studies with 5 IU, 10 IU or 20 IU Hylase “Dessau” on nine mono- and biphasic HAFs cross-linked by butanediol diglycidyl ether, but different technology and cross-linking degrees: (a) Juvéderm Volbella (15 mg HA/mL), Juvéderm Volift (17.5 mg HA/mL), and Juvéderm Voluma (20 mg HA/mL (monophasic, Allergan); (b) Restylane Fynesse (20 mg HA/mL), Restylane Refyne (20 mg HA/mL), and Restylane Volyme (20 mg HA/mL) (biphasic, Galderma); and (c) Belotero Balance (22.5 mg HA/mL), Belotero Intense (25.5 mg HA/mL), and Belotero Volume (26 mg HA/mL) (monophasic, Merz). Degradation was time- and hyaluronidase-dose dependent. Most fillers could be completely digested within 5–9 h with 20 IU Hyaluronidase “Dessau.” Only Belotero Volume showed a greater resistance to enzymatic degradation.17
Biphasic fillers of the Aliaxin line (IBSA Farmaceutici Italia S.R.L.; Lodi, Italy) containing diverse combinations of HA molecular weights from 500 to 2000 kDa were studied in vitro for resistance to ROS-mediated degradation. The H2O2/Cu2+ system was used for ROS generation. The storage modulus G’ was assessed over 10 min with constant strain and frequency and compared to HAF gels not exposed to ROS. HAF degradation was measured by G’ decrease starting four min after incubation. The gels showed different resistance to degradation by ROS. The HAF with the highest complex viscosity remained the most stable gel.18
Zerbinati et al.19 investigated the digestion of a polyethyleneglycol diglycidyl ether cross-linked HAF (Neauvia Intense; MatexLab SA, Lugano, CH) to hyaluronidase from bovine testis (Sigma) at 37°C in vitro. Degradation was measured by release of N-acetyl- D-glucosamine. The degradation was proportional to the incubation time and after 120 min a complete digestion was obtained.19 In a second set of experiments, HA-gels with different HA content (20–30 mg) and partially coupled with l-lysine were exposed to bovine hyaluronidase for up to 168 h. The kinetics of degradation were dependent on exposure time and HA content. The maximum degradation for the HA-gel with 30 mg/mL HA was 70.7% and 82.3% for the HA-gel with 20 mg/mL HA. The half maximal degradation was obtained after 58 h and 40 h, respectively.20
An animal model was established to evaluate the residence time of different HAFs that were native or fluorescein-labeled and injected subcutaneously in mice. 1,4-Butanediol diglycidyl ether-cross-linked HA 2.5% w/v was prepared for this experiment. Filler volumes were monitored by high-frequency ultrasound (HF-US) while fluorescence intensity was assessed by fluorescence living imaging. Both noninvasive methods revealed the same degradation kinetics for the tested products.21
Seven HAFs were investigated in hairless mice (SKH1- Hrhr): monophasic HAFs Neuramis Light Lidocaine, Neuramis Lidocaine, Neuramis Deep Lidocaine, Neuramis Volume Lidocaine, Neuramis Deep, and Juvéderm Ultra Plus XC, and biphasic Restylane LYFT with Lidocaine. The injection volume was 0.1 mL. The fillers were placed on the back of the animals. The researchers tested the susceptibility to hyaluronidase after 4 and 91 days residence. Ovine hyaluronidase (Liporase; DaeHan New Pharm Co., Ltd., Korea) was employed. A dosage of 30 IU resulted in a significant volume reduction in four days HAF implants within 1 h after hyaluronidase injection. A complete loss of surface projection was observed for Neuramis Light Lidocaine, Neuramis Lidocaine, and Juvéderm Ultra Plus XC within 60 min. Neuramis Deep Lidocaine, Neuramis Volume Lidocaine, Neuramis Deep, and Restylane LYFT with Lidocaine showed a complete loss of surface projection within 6 h. For HAF implants after 91 days two injections with 30 IU hyaluronidase were applied. All HAF implants showed a complete loss of surface projections between one hour and 24 h. This argues for a better response to hyaluronidase, when the tissue integration of the filler is still incomplete. That has implications for the management of unwanted adverse events with HAF where a rapid and complete dissolution of HAF is most crucial.22
An animal study from Italy investigated four different HAF with different HA content on mice, that is Viscoderm 0.8, Profhilo, Profhilo Structura, and Aliaxin GP (IBSA Farmaceutici Italia, Lodi, Italy) [Table 4]. The filler placement was on the back with volumes of 0.2 mL for each HAF. The deposited volume under the mouse skin was measured by high-frequency ultrasound imaging. Software generated 3D volumetric sonograms up to 33 weeks after filler placement. Degradation of HAF was dependent on HA content and cross-linking. In this study, HA hybrid complex 45 mg/mL was nearly completely decomposed after three hours of treatment with hyaluronidase in vitro, while cross-linked HA 25 mg/mL needed 24 h.23
| Product | HA content |
Structure | Complete degradation |
|---|---|---|---|
| Viscoderm 0.8 | 8 mg/mL | Linear | Week 4 |
| Profhilo | 32 mg/mL | Hybrid complex | Week 10 |
| Profhilo Stuctura | 45 mg/mL | Hybrid complex | Week 29 |
| Aliaxin GP | 25 mg/mL | Cross-linked HA | Week 33 |
HAF: Hyaluronic acid filler , HA:Hyaluronic acid
Degradation of intradermal HAFs in vivo has been prospectively investigated in nine subjects. The arms were injected with 0.2 mL of Restylane-L, Juvéderm Ultra, and Juvéderm Voluma subcutaneously. Thereafter hyaluronidase (Hylenex) was injected at varying doses from 2.5 to 20 IU. The effect was compared to a control site. Diameter, elevation, and firmness were followed for up to 2 weeks. The most significant changes for HAFs occurred between 30 min and 3 h after injection of hyaluronidase followed by a gradual degradation through week 2. A dose–response relationship was observed.24
An example of induced degradation of HAF is shown in Figure 1. For various hyaluronidase products see Table 5.
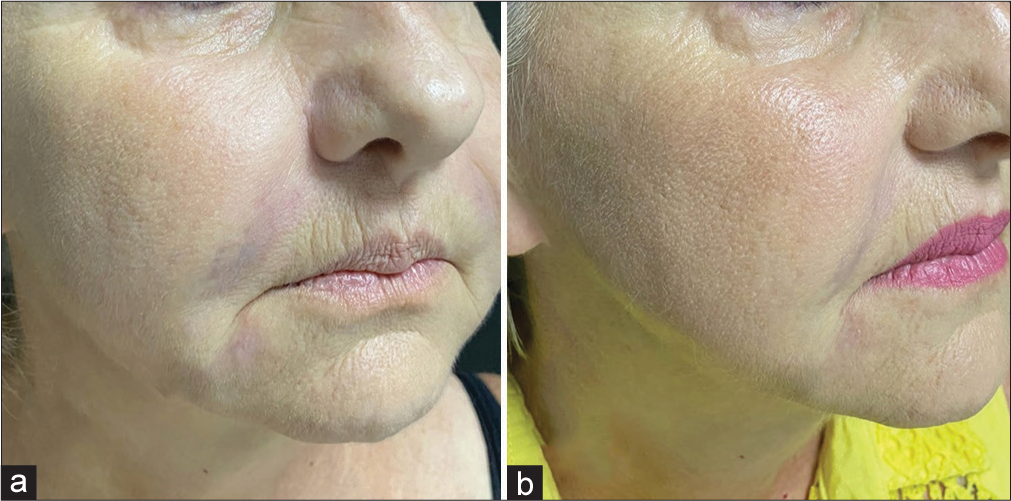
- Induced degradation of a temporary filler in a female patient (61-year-old). (a) Formation of nodules and hardened areas in the nasolabial folds, mouth commissure, and mandibular contour after the application of hyaluronic acid on the face a week ago. (b) Two days after application of hyaluronidase in compromised areas: Complete resolution of the nodules and the overcorrection.
| Product | Remarks | Typical amount per Injection (in units/mL) |
|---|---|---|
| Amphadase | Amphastar Pharmaceuticals, Rancho Cucamonga, CA | 75–150 USA (Bovine) |
| Desinfitral | Aesthetic Dermal, Girona, Spain | 150 (Ovine) |
| Hyaluronidase | Lee Pharmacy, Fort Smith, AZ, USA | 75 |
| Hylase “Dessau” | Earlier: Riemser Pharma, Greifswald, Germany (Bovine) | 20–30 Now: Esteve Pharmaceuticals, Berlin, Germany |
| Hylenex | Baxter Healthcare Corporation, Deerfield, IL, USA | 75–150 and Halozyme Therapeutics, San Diego, CA, USA (Recombinant) |
| Vitrase | Earlier: IstaPharmaceuticals, Irvine, CA, USA (Ovine) | 75 Now: Bausch & Lomb; Vaughan, Ontario, Canada |
HAF: Hyaluronic acid filler
POLYLACTIC ACID FILLERS
Polylactic acid (PLA) fillers are biocompatible and biodegradability. They are further characterized by the possible formation of both amorphous and semicrystalline phases. Due to thermal factors, photodegradation and oxidation PLA changes mechanical and optical properties.
PLA fillers are composed of PLA microparticles and carriers, such as mannitol or sodium carboxymethylcellulose. PLA exists in two stereoisomeric forms, namely l-lactic and D-lactic acid. Poly(D,l-lactic acid) is completely amorphous in contrast to l-lactic acid and D-lactic acid. Sculptra and Gana V–two l-lactic PLA fillers—show a crystallinity degree of 64% and 72%. PLA degradation is induced by hydrolysis due to water sorption in the microparticles itself. This results in breakage of the ester linkages in the main chain of the polymer molecule. The soluble degradation products are lactic acid and its oligomers. The rate of degradation depends upon various factors including the l-to-D-isomer ratio, molecular weight, crystallinity, size, shape, porosity, and morphology of the microparticles among others. The rate of lactic acid formation during degradation of PLA filler can modulate fibroblast activity and collagen synthesis [Table 6].25
| Product | Microparticles | Carrier | MW (kDa) | Degradation period | |
|---|---|---|---|---|---|
| Claims* | In vitro | ||||
| Sculptra | 150 mg poly (l-PLA) | CMC/mannitol | 78 | 24 months | 24 months |
| Repart PLA | 154 mg poly (D, l-PLA) | CMC/mannitol | 107 | 24 months | 10 months |
| AestheFill | 154 mg poly (D, l-PLA) | CMC | 80 | 24 months | 12 months |
| Gana V | 210 mg poly (l-PLA) | CMC/mannitol | 114 | 27.6 months | 24 months |
PLA: Polylactic acid, CMC: Carboxymethylcellulose, MW: Molecular weight. *According to the manufacturer
Hydrolytic degradation of PLA filler is a main mechanism of biodegradation. Tissue water diffuses into the microspheres, causing hydrolysis at the surface and throughout the particle volume depending on particle size, shape, and porosity. The penetrating water rapidly creates a negative gradient of water concentrations from the surface to the center, but this gradient vanishes since the diffusion of water is faster than the degradation rates. The degradation of microspheres is monomodal. The molecular weight of PLA decreases until it becomes completely soluble in water.26
Degradation of microspheres was studied in vitro. The particles were suspended either in distilled water (AestheFill) or phosphate-buffered saline at 37°C for 9 months (the remaining PLAs). Samples were analyzed for particle size and morphology by scanning electron microscopy. Most of the PLA fillers tested demonstrated a monomodal and gradually shift toward lower molecular weights suggesting a bulk degradation. Gana V, however, developed a second peak of particles with increased dispersity, arguing for a heterogeneous degradation accompanied by the formation of low-molecular weight oligomers. The degradation rate was highest with Repart PLA, followed by AestheFill, Sculptra, and Gana V.25
CALCIUM HYDROXYLAPATITE FILLER
Calcium hydroxylapatite (CaHA) fillers are semipermanent and biostimulatory. They have high elasticity and viscosity. The volumizing and sculpturing effect is longer lasting compared to HAF. Nevertheless, the product is completely biodegradable and not permanent. The CaHA microspheres are degraded into calcium and phosphate ions.26,27
The first registered HAF filler was Radiance FN followed by Radiesse which is now more than 10 years on the market.28,29 Radiesse is a biphasic filler consisting of 30% synthetic CaHA microspheres suspended in aqueous carboxymethylcellulose gel and glycerin. The CaHA microspheres are thought to stimulate collagen synthesis by fibroblast activation. A different approach is the incorporation of low concentration CaHA in HAF.30 But also in the low concentration range, CaHA seems to be stimulatory for collagen production.31,32 Currently available products are listed in Table 7.
| Product | Company | Characteristics |
|---|---|---|
| Radiesse | Merz Aesthetics | 30% CaHA microspheres 25–45 μm, round shaped, nonporous, carriers: glycerin and sodium-carboxymethylcellulose |
| Crystalis | Luminera Derm Ltd. | 55.7% CaHA microspheres 25–45 μm, round shaped, nonporous, carriers: glycerin and sodium-carboxymethylcellulose |
| Harmony CA | Luminera Derm Ltd. | 55.7% CaHA microspheres 25–45 μm, round shaped, nonporous, carrier: nonanimal, highly crosslinked hyaluronic acid |
| Neauvia Stimulate | MatexLab SA | 1% CaHA microspheres in HAF (HA 26 mg/mL, polyethylene glycol cross-linked) |
HAF: Hyaluronic acid filler, HA:Hyaluronic acid, CaHA: Calcium hydroxylapatite
In a preclinical study Iafisco et al.33 investigated the role of shape, size, and crystallinity for decomposition of CaHA nanocrystals in Friend Virus B (FVB) mice. They synthesized poorly crystalline and highly crystalline hydroxylapatite. The resulting clusters of poorly crystalline microparticles had larger dimensions (30 µm) than the highly crystalline samples (3 µm). The highly crystalline microparticles were completely degraded within 4 weeks after implantation while the larger poor crystalline particle clusters were still present 8 weeks after implantation.33 The findings underline the role of size and structure of CaHA particles for their degradation. Osteolytic enzymes such as ubiquitin-specific proteases, matrix metalloproteinases or cysteine protease cathepsin K are involved in spontaneous degradation.
In a proof-of-concept study, 12 cadaveric porcine skin samples were injected with CaHA (0.4–0.8 mL) and randomized to (a) intralesional injection of 0.2 mL sodium thiosulfate (12.5 g/50 mL), (b) topical sodium metabisulfite 25% gel with occlusion, or (c) both treatments. Control samples were not treated after CaHA injection. Histologic analysis was done 24 h later. Both intralesional sodium thiosulfate (STS) alone or combined with topical sodium metabisulfite 25% gel under occlusion completely dissolved CaHA microparticles, while topical sodium metabisulfite alone was of limited efficacy.34 In contrast to the effect on porcine skin, intravascular CaHA particles did not respond as well suggesting that intravascular sodium thiosulfate is not sufficient to rapidly dissolve intravascular CaHA material.35
The biodegradation of Radiesse had been evaluated in vivo using computed tomographic imaging before and after treatment. In a study with 58 patients suffering from HIV-associated lipoatrophy or pronounced nasolabial folds only residual amounts of CaHA could be observed 12 months after the initial injection. However, the esthetic improvements remained evident at 12 months which argues for a scaffold function of this filler type.36 CaHA microspheres were detected in histologic specimen of Mohs surgery up to six years after filler injection to correct nasolabial folds suggesting a much longer persistence and resistance to biodegradation in certain regions and selected patients.37
POLYALKYLIMIDE HYDROGEL FILLER
Polyalkylimide hydrogel (PAH) consists of a polyacrylic hydrogel containing alkyl imide-amide groups (4%) and water (96%) (Bio-Alcamid, Polymekon, Brindisi, Italy). It is nonresorbable and biocompatible and has been marketed from 2001 onwards.38 Migration even years after injection and inflammatory reactions have been reported.39
Extruded filler material of 34 patients with such complications was analyzed for possible modifications of the filler material. The authors reported that the PAH changed macroscopically and microscopically suggesting a certain degree of degradation in the human body.40
Inducible degradation is unknown with PAH fillers.
POLYMETHACRYLATE FILLER
There is a wide array of polymethacrylate filler (PMMA)-based injectable products available which have been approved, including PMMA in collagen (Artefill, Suneva Medical Inc., San Diego, CA, USA; Artecoll (Hafod Bioscience BV, Nijmegen, The Netherlands), PMMA in carboxyglutamate (Metacrill, Nutricel, Rio de Janeiro, RJ, Brazil), and PMMA in carboxymethylcellulose (Newplastic, Lebon Produtos Químicos e Farmacêuticos, Porto Alegre, RS, Brazil). Other hydroxyethylmethacrylate particles suspended in hyaluronic acid (DermaLive, Dermatech, Paris, France) and polyvinyl hydroxide microspheres suspended in polyacrylamide gel (Evolution, ProCytech SA, Bordeaux, France), have been withdrawn from the market.2
ArteFill consists of PMMA microspheres (20%) measuring 30–50 μm in diameter, suspended in 3.5% bovine collagen solution (80%) and 0.3% lidocaine, while Dermalive contains 40% PMMA, 20–120 µm in size, and 60% HAF. A histologic study at 3 months and 10 years after injection of ArteFill demonstrated encapsulation of PMMA particles by collagen fibers. No signs of degradation have been noted.41 After injection of 0.1 mL in the forearm of one proband PMMA particles (Dermalive) had disappeared clinically at 6 months, while PMMA microspheres (Artecoll)—the predecessor of ArteFill—remained.42
The difference of persistence of nonabsorbable filler material might be related to the particle size, since in vitro experiments demonstrated that various cells (U-937 cells, keratinocytes, and Langerhans cells) can phagocytose PMMA particles of less than or equal to 20 µm.43
There are limited data indicating a successful inducible degradation of PMMA microspheres. Intralesional application of 1064 nm neodymium: Yttrium aluminum garnet (YAG) laser therapy is beneficial to extrude the filler material in case of inflammatory or cosmetic complications. The laser energy is delivered through a 300–600-µm fiber optic, embedded in a stainless-steel micro-cannula. The emitted laser energy can cause fragmentation of PMMA by (1) charge-directed fragmentations, (2) charge-remote rearrangements, and (3) charge-remote fragmentations via radical intermediates.44,45 An example of treatment is shown in Figure 2.
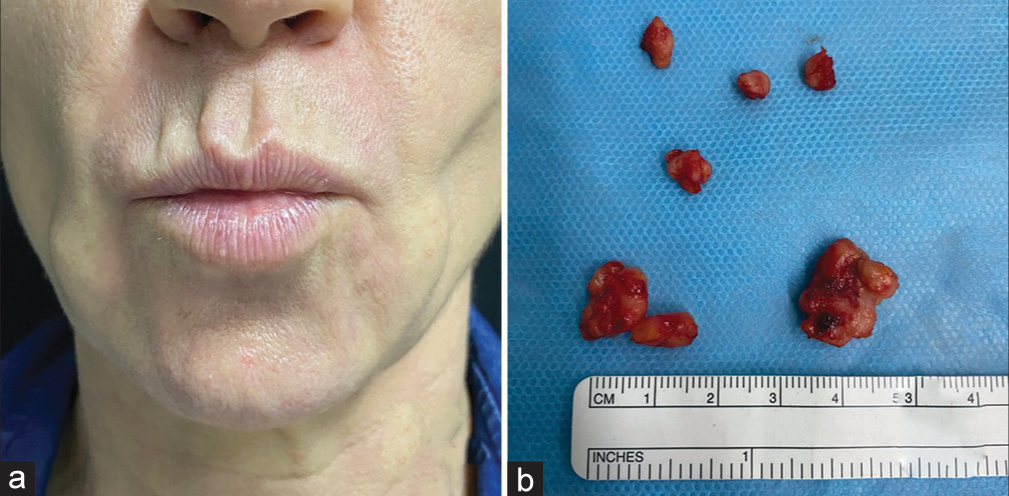
- Female patient, 53-year-old. Polymethylmethacrylate was injected 12 years ago in the face. (a) Foreign body granulomas on the face. (b) Macroscopic specimen removed after intralesional laser treatment.
CONCLUSION
There is a broad range of soft tissue fillers for esthetic dermatology on the market. Degradation, either spontaneous or induced, varies considerably between the products. While spontaneous degradation is responsible for the limited efficacy of temporary and semipermanent filler products, induced degradation is important to combat filler complications such as nodule formation or vascular compromise. In vitro and in vivo data on degradation are available for HAFs, while for most other products the data situation is still patchy. Various products of hyaluronidase and typical amounts (in units/mL) used for HAF dissolution are summarized for orientation. Dependent upon the clinical situation, several injections of the enzyme may be necessary to obtain the best outcome. Data on degradation of fillers are as important as data on biocompatibility and toxicity to increase consumer safety.
Authors’ contributions
Uwe Wollina and Alberto Goldman contributed equally to concepts, design, definition of intellectual content, literature search, data acquisition, data analysis, manuscript preparation, manuscript editing, and manuscript review. Uwe Wollina is the guarantor.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Treating aging changes of facial anatomical layers with hyaluronic acid fillers. Clin Cosmet Investig Dermatol. 2021;14:1105-18.
- [CrossRef] [PubMed] [Google Scholar]
- Overview of deep dermal fillers. Facial Plast Surg. 2019;35:224-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hyaluronic acid dermal fillers: Safety and efficacy for the treatment of wrinkles, aging skin, body sculpturing and medical conditions. Clin Med Rev Therapeutics. 2011;3:107-21.
- [CrossRef] [Google Scholar]
- Physiochemical properties and application of hyaluronic acid: A systematic review. J Cosmet Dermatol. 2016;15:520-6.
- [CrossRef] [PubMed] [Google Scholar]
- Poly(a-hydroxy acid) based polymers: A review on material and degradation aspects. Polym Degrad Stab. 2017;144:520-35.
- [CrossRef] [Google Scholar]
- The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499-508.
- [CrossRef] [PubMed] [Google Scholar]
- Hyaluronidase: From clinical applications to molecular and cellular mechanisms. Eur J Med Res. 2016;21:5.
- [CrossRef] [PubMed] [Google Scholar]
- Durability, behavior, and tolerability of 5 hyaluronidase products. Dermatol Surg. 2018;44:S42-50.
- [CrossRef] [PubMed] [Google Scholar]
- The kinetics of reversible hyaluronic acid filler injection treated with hyaluronidase. Dermatol Surg. 2017;43:841-7.
- [CrossRef] [PubMed] [Google Scholar]
- Degradation of hyaluronic acid fillers using hyaluronidase in an in vivo model. J Drugs Dermatol. 2018;17:548-53.
- [Google Scholar]
- Modulation of biomechanical properties of hyaluronic acid hydrogels by crosslinking agents. J Biomed Mater Res A. 2015;103:3072-80.
- [CrossRef] [PubMed] [Google Scholar]
- Ex vivo magnetic resonance imaging using hyaluronic acid fillers: Differences between monophasic and biphasic fillers. Skin Res Technol. 2018;24:16-9.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term MRI follow-up of hyaluronic acid dermal filler. Plast Reconstr Surg Glob Open. 2022;10:e4252.
- [CrossRef] [PubMed] [Google Scholar]
- Model-based prediction to evaluate residence time of hyaluronic acid based dermal fillers. Pharmaceutics. 2021;13:133.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative analysis of commercial dermal fillers based on crosslinked hyaluronan: Physical characterization and in vitro enzymatic degradation. Polym Degrad Stab. 2011;96:630-6.
- [CrossRef] [Google Scholar]
- Quantifying the digestion of cross-linked hyaluronic acid fillers with hyaluronidase. Dermatol Surg. 2021;47:1233-6.
- [CrossRef] [PubMed] [Google Scholar]
- Time-and dose-dependent effects of hyaluronidase on the degradation of different hyaluronan-based fillers in vitro. Plast Reconstr Surg. 2023;151:560-7.
- [CrossRef] [PubMed] [Google Scholar]
- Hyaluronan dermal fillers: Efforts towards a wider biophysical characterization and the correlation of the biophysical parameters to the clinical outcome. Clin Cosmet Investig Dermatol. 2020;13:87-97.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro evaluation of the sensitivity of a hyaluronic acid PEG cross-linked to bovine testes hyaluronidase. Open Access Maced J Med Sci. 2018;6:20-4.
- [CrossRef] [PubMed] [Google Scholar]
- Physico-chemical characterization and in vitro biological evaluation of a bionic hydrogel based on hyaluronic acid and l-lysine for medical applications. Pharmaceutics. 2021;13:1194.
- [CrossRef] [PubMed] [Google Scholar]
- A novel animal model for residence time evaluation of injectable hyaluronic acid-based fillers using high-frequency ultrasound-based approach. Clin Cosmet Investig Dermatol. 2018;11:339-46.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative analysis of hyaluronidase-mediated degradation among seven hyaluronic acid fillers in hairless mice. Clin Cosmet Investig Dermatol. 2021;14:241-8.
- [CrossRef] [PubMed] [Google Scholar]
- Elucidations on the performance and reversibility of treatment with hyaluronic acid based dermal fillers: In vivo and in vitro approaches. Clin Cosmet Investig Dermatol. 2022;15:2629-40.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective in vivo evaluation of three different hyaluronic acid gels to varying doses of hyaluronidase with long-term follow-up. J Plast Reconstr Aesthet Surg. 2021;74:874-80.
- [CrossRef] [PubMed] [Google Scholar]
- Hydrolytic degradation of devices based on poly(DL-lactic acid) size-dependence. Biomaterials. 1995;16:305-11.
- [CrossRef] [PubMed] [Google Scholar]
- Physicochemical characteristics and hydrolytic degradation of polylactic acid dermal fillers: A comparative study. Cosmetics. 2023;10:110.
- [CrossRef] [Google Scholar]
- Calcium hydroxylapatite filler: An overview of safety and tolerability. J Drugs Dermatol. 2013;12:996-1002.
- [Google Scholar]
- Calcium hydroxylapatite microspheres-Biocompatibility and clinical effects. Georgian Med News. 2018;278:62-8.
- [Google Scholar]
- Radiance FN: A new soft tissue filler. Dermatol Surg. 2004;30:764-8. discussion 768
- [CrossRef] [PubMed] [Google Scholar]
- Calcium hydroxylapatite: Over a decade of clinical experience. J Clin Aesthet Dermatol. 2015;8:38-49.
- [Google Scholar]
- In vitro evaluation of the biosafety of hyaluronic acid peg cross-linked with micromolecules of calcium hydroxyapatite in low concentration. Open Access Maced J Med Sci. 2018;6:15-9.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro evaluation of collagen production on human fibroblasts treated with hyaluronic acid PEG cross-linked with micromolecules of calcium hydroxyapatite in low concentration. J Biol Regul Homeost Agents. 2017;31:87-90.
- [Google Scholar]
- The cooperative effect of size and crystallinity degree on the resorption of biomimetic hydroxyapatite for soft tissue augmentation. Int J Artif Organs. 2010;33:765-74.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro analysis of the degradation of calcium hydroxylapatite dermal filler: A proof-of-concept study. Dermatol Surg. 2018;44:S5-9.
- [CrossRef] [PubMed] [Google Scholar]
- Intraarterial degradation of calcium hydroxylapatite using sodium thiosulfate-an in vitro and cadaveric study. Aesthet Surg J. 2021;41:NP226-36.
- [CrossRef] [PubMed] [Google Scholar]
- Radiographic and computed tomographic studies of calcium hydroxylapatite for treatment of HIV-associated facial lipoatrophy and correction of nasolabial folds. Dermatol Surg. 2008;34:S78-84.
- [CrossRef] [Google Scholar]
- Calcium hydroxylapatite tissue filler discovered 6 years after implantation into the nasolabial fold: Case report and review. Dermatol Surg. 2009;35:375-9.
- [CrossRef] [PubMed] [Google Scholar]
- a novelty for reconstructive and cosmetic surgery. Ital J Anat Embryol. 2002;107:209-14.
- [Google Scholar]
- Complications after treatment with polyalkylimide. Dermatol Surg. 2009;35:1625-8.
- [CrossRef] [PubMed] [Google Scholar]
- Polyalkylimide: A nonstable filler over time. Dermatol Surg. 2018;44:563-7.
- [CrossRef] [PubMed] [Google Scholar]
- ArteFill permanent injectable for soft tissue augmentation: I. Mechanism of action and injection techniques. Aesthetic Plast Surg. 2010;34:264-72.
- [CrossRef] [PubMed] [Google Scholar]
- Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003;27:354-66. discussion 367
- [CrossRef] [PubMed] [Google Scholar]
- Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatol Surg. 2002;28:484-90.
- [CrossRef] [PubMed] [Google Scholar]
- Polymethylmethacrylate-induced nodules of the lips: Clinical presentation and management by intralesional neodymium: YAG laser therapy. Dermatol Ther. 2019;32:e12755.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional neodymium YAG laser to treat complications of polymethylmethacrylate. Open Access Maced J Med Sci. 2018;6:1636-41.
- [CrossRef] [PubMed] [Google Scholar]






