Translate this page into:
Syringocystadenoma papilliferum with tubular apocrine adenoma without nevus sebaceous – A rare composite tumor of pinna presenting in a blaschkoid pattern
*Corresponding author: Ravi Hari Phulware, Department of Pathology and Laboratory Medicine, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India. ravipaarti@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Behera P, Arora P, Bhardwaj M, Phulware RH. Syringocystadenoma papilliferum with tubular apocrine adenoma without nevus sebaceous – A rare composite tumor of pinna presenting in a blaschkoid pattern. J Cutan Aesthet Surg. doi: 10.25259/jcas_34_24
Abstract
Syringocystadenoma papilliferum (SCAP) is an uncommon benign adnexal tumor, most commonly involving the head-and-neck region. It usually occurs singly or may be seen in association with other tumors. The association of tubular apocrine adenoma (TAA) with SCAP in the background of nevus sebaceous (NS) on the scalp is well documented. However, the coexistence of these two tumors without pre-existing NS is uncommon. We report a case of blaschkoid SCAP associated with TAA over the right pinna and adjacent scalp without pre-existing NS in a young male patient.
Keywords
Tubular apocrine adenoma
Syringocystadenoma papilliferum
Nevus sebaceous
Adnexal neoplasms
INTRODUCTION
Syringocystadenoma papilliferum (SCAP) is a benign tumor of disputed histogenesis.1 It is thought to arise from either pluripotent appendageal cells.2 SCAP associated with tubular apocrine adenoma (TAA) in the background of nevus sebaceous (NS) on the head-and-neck area has been reported in the past.2 However, the coexistence of these two tumors without an underlying NS has rarely been reported. Herein, we report an unusual case of SCAP associated with TAA on the right pinna and scalp without pre-existing NS in a young male.
CASE REPORT
A 15-year-old male presented with multiple asymptomatic warty lesions over the posterior aspect of the right ear since birth. There was a history of an increase in the size of the lesions in the past few years. The patient reported occasional episodes of pus discharge from the lesions. Cutaneous examination revealed multiple skin-colored to erythematous papules and nodules arranged in a linear array along Blaschko lines over the posterior aspect of the right pinna extending to the adjacent scalp below the hairline. There was oozing and crusting in a few areas [Figure 1]. Punch biopsy from the lesion revealed two distinctly different pathologies [Figure 2a]. The epidermis showed irregular acanthosis along with a cystic invagination into the upper dermis, lined by squamous epithelium near the epidermal surface and two layers of glandular epithelium (tall columnar luminal cells and cuboidal basal cells) below. Few papillary projections lined by similar types of two rows of epithelial cells protruded into the lumen of the cystic invagination [Figure 2b].
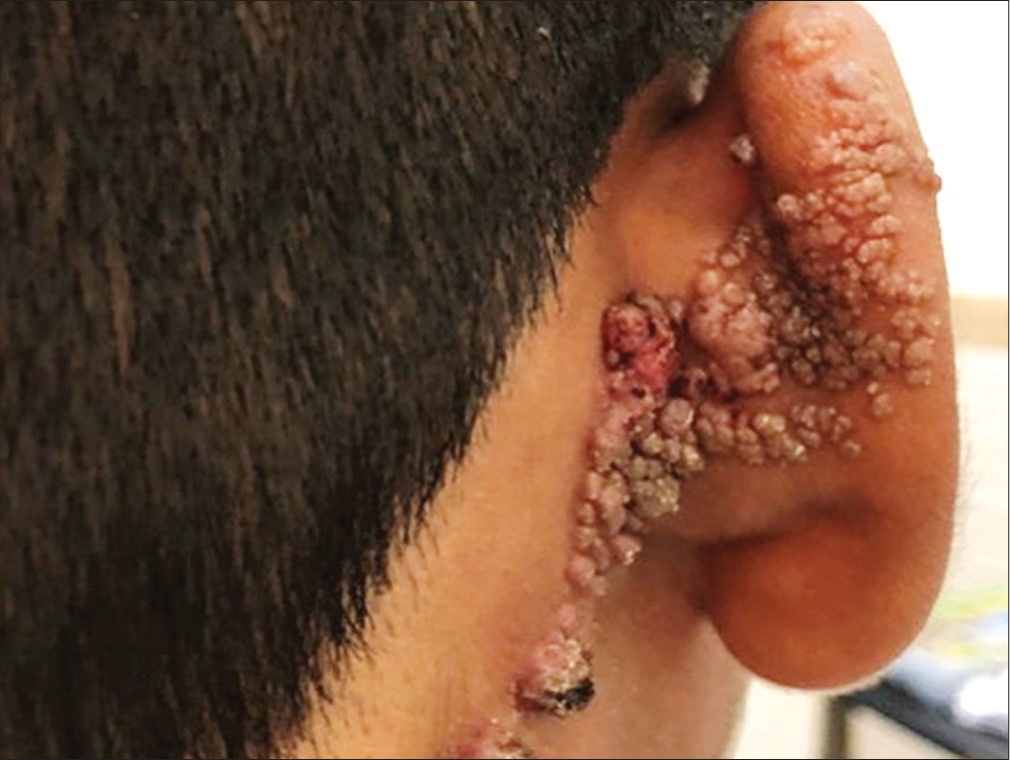
- Coalescing erythematous papules following lines of Blaschko.
![Photomicrographs of tumor, (a) syringocystadenoma papilliferum (SCAP) in the upper dermis and tubular apocrine adenoma in deep dermis (Hematoxylin and eosin [H&E]; ×200), (b) acanthotic epidermis with underlying cystic invaginations thrown into papillae, lined by two layers of epithelium, inner columnar cells and outer layer of cuboidal cells; suggestive of SCAP (H&E; ×100), (c) papillae lined by double-layered epithelium with intervening stroma showing inflammatory infiltrates, predominantly plasma cells (H&E; ×400), and (d) variably dilated round to oval tubules in deep dermis lined by apocrine cells, some containing eosinophilic amorphous secretions (H&E, ×200). (e) Luminal cells of tubular apocrine adenoma showing positive reaction to Gross cystic disease fluid protein 15 (GCDFP-15) (×200) and (f) Negative staining result to GCDFP-15 in syringocystadenoma papilliferum (×100).](/content/173/2024/0/1/img/JCAS-34-24-g002.png)
- Photomicrographs of tumor, (a) syringocystadenoma papilliferum (SCAP) in the upper dermis and tubular apocrine adenoma in deep dermis (Hematoxylin and eosin [H&E]; ×200), (b) acanthotic epidermis with underlying cystic invaginations thrown into papillae, lined by two layers of epithelium, inner columnar cells and outer layer of cuboidal cells; suggestive of SCAP (H&E; ×100), (c) papillae lined by double-layered epithelium with intervening stroma showing inflammatory infiltrates, predominantly plasma cells (H&E; ×400), and (d) variably dilated round to oval tubules in deep dermis lined by apocrine cells, some containing eosinophilic amorphous secretions (H&E, ×200). (e) Luminal cells of tubular apocrine adenoma showing positive reaction to Gross cystic disease fluid protein 15 (GCDFP-15) (×200) and (f) Negative staining result to GCDFP-15 in syringocystadenoma papilliferum (×100).
The stroma was composed of predominantly plasma cells, along with a few lymphocytes and neutrophils [Figure 2c]. The deeper portion consisted of a few circumscribed lobules of well-differentiated tubular structures with two or more layers of epithelial cells [Figure 2d]. The outer layer was composed of flattened cuboidal cells, whereas the inner layer was composed of columnar cells, which also showed decapitation secretion. Prominent apocrine differentiation was noted. Some of the tubules were filled with amorphous eosinophilic material. The findings in the upper portion of the lesion were interpreted as SCAP, and those in the deeper portion suggested a histological diagnosis of TAA [Figure 2e and f]. There were no features of NS in any of the sections examined.
DISCUSSION
SCAP is a benign sweat gland tumor that usually presents at birth or develops in early childhood. It presents as solitary or multiple papules in a linear distribution or as a plaque. The lesions often increase in size at puberty, become papillomatous, and are usually crusted. Scalp and face are the most common sites of involvement, but it has been seen that 25% of the cases can occur in other areas of the body (e.g., trunk, upper arms, vulva, and thighs).3 Histopathologically, SCAP consists of one or more cystic invaginations from the surface epithelium into the underlying dermis. The upper portion of invaginations has a keratinizing squamous cell lining similar to the epidermis. The lower portion of the invaginations gives rise to numerous papillary projections that extend into the lumina of the invaginations. These papillary projections, as well as the lower portion of invaginations, are lined by two layers of glandular epithelium; the outer, cuboidal, and the inner, columnar cell layer shows evidence of decapitation secretion. The papillomatous projections are supported by plasma cell-rich fibrovascular cores.3,4 Although most lesions of SCAP appear to be of apocrine origin, some exhibit eccrine derivation.4 Hence, it has been postulated that SCAP arises from undifferentiated cells, which have the potential to exhibit both apocrine and eccrine modes of epithelial secretion.
TAA is also a rare sweat gland tumor, first described by Landry and Winkelmann in 1972.1 It is usually found in the scalp of females as a solitary nodule,2 unlike our case, who was an adult male with multiple lesions on the pinna. Histopathologically, exemplary cases of TAAs are composed of well-defined lobules in the dermis and occasionally in the subcutis. These lobules are made up of variably dilated tubules that are usually lined by two layers of epithelial cells: peripheral cuboidal cells and luminal columnar cells layer.5 In some tubules, the columnar cells show decapitation secretion. Epithelial papillary projections devoid of central stroma can be seen in the interior of these tubules. The stroma surrounding the tubules shows collagen condensation and fibrosis, with minimal lymphohistiocytic infiltration. Plasma cells are typically few or absent. The epidermis may show areas of pseudoepitheliomatous hyperplasia. The tumor can communicate with the epidermis by means of comedone-like structures.5 Ishiko et al. reviewed 19 cases of TAAs described in the literature and found that in 10 of the TAA cases, the tumor was connected to the overlying epidermis.6 Hence, there is a significant morphologic overlap between SCAP and TAA.4 Some of the points which favor TAA over SCAP include the presence of fibrous connective tissue stroma with no or rare plasma cells along with the absence of cystically dilated epidermal invaginations and thick dermal papillae.5
The first case of SCAP with TAA, published in English literature, was by Toribio et al. in the year 1987.5 Since then, very few similar cases have been described in the literature, with rare cases occurring in the absence of NS. Ishiko et al. stated that the combination of SCAP and TAA occurs only in a preexisting NS.6 We found 15 cases of SCAP associated with TAA,7 out of which only five cases described the absence of preexisting NS [Table 1].
In our patient also, the lesions were not associated with NS, although they were located on the posterior aspect of the right pinna and adjacent scalp, which is otherwise a common site for NS. Recently, Khullar et al. also reported a case of SCAP with TAA in the absence of NS.8 According to Lee et al., the tubular structures in the lower portion of the lesion are the ectopic apocrine glands in an underdeveloped NS.3 They proposed this considering the pre-pubertal status of their patient. Furthermore, Requena et al. suggested that the deep dermal tubular component may be a part of SCAP.9 However, the tubular structures in this case were numerous and consisted of well-differentiated tubular islands without plasma cells and so were characteristic of TAA.
| Reference | Age/sex | Location | Clinical features | Size | Nevus sebaceous |
|---|---|---|---|---|---|
| Lee HJ et al.3(2011) (PMID: 22148038) |
12/F | Lower dorsum | An erosive crusted papillomatous plaque | 1.8 × 2.5 cm | Absent |
| Toribo et al.5(1987) (PMID: 3036918) |
33/M | Scalp | An exudative round pedunculated lesion | 2 cm | Not mentioned |
| Ishiko et al.6(1993) (PMID: 8238787) |
75/M | Scalp | A verrucous tumour located on depigmented plaque | 1.1 cm | Present |
| Leda et al.7(2017) (PMID: 29166517) |
14/M | Dorsum | A whitish nodule with central ulceration | Not mentioned | Absent |
| Khuller et al.8(2018) | 20/M | Face | skin-coloured to ery- thematous papules coalescing to form two plaques | 3 × 2 cm | Absent |
| Malhotra et al.10(2011) | 52/M | Scapula | Ulcerated, verrucous growth | 3 × 2 cm | Present + SCC |
| Ansai et al.11(1989) (PMID: 2551940) |
22/M | Scalp | A pedunculated tumor with lobulated and erosive surface | 1.3 × 1.6 × 1cm | Not mentioned |
| Aktepe et al.12(2003) (PMID: 12639465) |
19/M | Scalp | A pinkish, verrucous plaque | 3 × 1cm | Present |
| Ahn et al.13(2004) | 52/M | Scalp | A dome shaped nodule | 1.5 cm | Present |
| Lee CK et al.14(2005) (PMID: 15235197) |
74/F | External auditory canal | An indurated tumour with central ulceration | 1.5 × 1 cm | Absent |
| Yamane et al.15(2007) | 77/F | Mammary region | Linear plaques, ulcerated pedunculated nodule | 10 × 4 cm | Present |
| Vazmitel et al.16(2008) | 61/M | Scalp | Ulcerated plaque | Not mentioned | Present |
| Kim et al.17(2010) (PMID: 20711270) |
40/M | Scalp | A pedunculated nodule | 2.5 cm | Present |
| Yoon JH et al.18(2011) (PMID: 22148043) |
59/M | Calf | Itchy, erythematous and lobulated nodule | 1.3 × 1.2 cm | Absent |
| Epstein et al.19(1990) | 15/M | Chest | Linear grouped shiny erythematous papules | 5 × 1 cm | Not mentioned |
| Present case | 15/M | Pinna +Scalp | Multiple papules and nodules with crust | 0.2-1.5cm | Absent |
SCC- Squamous cell carcinoma, M- Male, F- Female, TAA: Tubular apocrine adenoma
Squamous cell carcinoma is a rare complication in longstanding cases associated with NS.10 To prevent such malignant transformations, close surveillance is advised, along with prophylactic excision of all new growths.10
CONCLUSION
We present a rare case of a SCAP that was associated with TAA without NS. The points that make this case more interesting are the clinical presentation, which showed a blaschkoid distribution of the lesions on the posterior aspect of the pinna, and its non-association with NS.
Authors’ contributions
Conceptualization: Pushpanjali Behera, Minakshi Bhardwaj and Ravi Hari Phulware; formal analysis writing original draft preparation: all authors; writing-review and editing: Pushpanjali Behera, Pooja Arora, Minakshi Bhardwaj, and Ravi Hari Phulware; supervision: Ravi Hari Phulware, Pooja Arora, and Minakshi Bhardwaj. All authors have read and agreed to the published version of the manuscript.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- An unusual tubular apocrine adenoma. Arch Dermatol. 1972;105:869-79.
- [CrossRef] [PubMed] [Google Scholar]
- Tumors arising in nevus sebaceus: A study of 596 cases. J Am Acad Dermatol. 2000;42:263-8.
- [CrossRef] [PubMed] [Google Scholar]
- Syringocystadenoma papilliferum of the back combined with a tubular apocrine adenoma. Ann Dermatol. 2011;23:151-4.
- [CrossRef] [PubMed] [Google Scholar]
- Tubular adenoma and syringocystadenoma papilliferum: A reappraisal of their relationship. An interobserver study of a series by a panel of dermatopathologists. Am J Dermatopathol. 2007;29:256-63.
- [CrossRef] [PubMed] [Google Scholar]
- Is tubular apocrine adenoma a distinct clinical entity? Am J Dermatopathol. 1993;15:482-7.
- [CrossRef] [PubMed] [Google Scholar]
- Syringocystadenoma papilliferum combined with a tubular apocrine adenoma. An Bras Dermatol. 2017;92:721-3.
- [CrossRef] [PubMed] [Google Scholar]
- Blaschkoid distribution of composite syringocystadenoma papilliferum and tubular apocrine adenoma without naevus sebaceous. Clin Exp Dermatol. 2019;44:438-40.
- [CrossRef] [PubMed] [Google Scholar]
- Neoplasms with apocrine differentiation Philadelphia, PA: Lippicott-Raven; 1998. p. :22.
- [Google Scholar]
- Squamous cell carcinoma, syringocystadenoma papilliferum and apocrine adenoma arising in a nevus sebaceous of Jadassohn. Indian J Pathol Microbiol. 2011;54:225-6.
- [CrossRef] [PubMed] [Google Scholar]
- A case of tubular apocrine adenoma with syringocystadenoma papilliferum. J Cutan Pathol. 1989;16:230-6.
- [CrossRef] [PubMed] [Google Scholar]
- Tubular apocrine adenoma in association with syringocystadenoma papilliferum. Dermatol Online J. 2003;9:7.
- [CrossRef] [PubMed] [Google Scholar]
- A case of tubular apocrine adenoma with syringocystadenoma papilliferum arising in nevus sebaceus. J Dermatol. 2004;31:508-10.
- [CrossRef] [PubMed] [Google Scholar]
- Tubular apocrine adenoma with syringocystadenoma papilliferum arising from the external auditory canal. J Laryngol Otol. 2005;119:1004-6.
- [CrossRef] [PubMed] [Google Scholar]
- Naevus sebaceus on the female breast accompanied with a tubular apocrine adenoma and a syringocystadenoma papilliferum. Br J Dermatol. 2007;156:1397-9.
- [CrossRef] [PubMed] [Google Scholar]
- Syringocystadenoma papilliferum with sebaceous differentiation in an intradermal tubular apocrine component. Report of a case. Am J Dermatopathol. 2008;30:51-3.
- [CrossRef] [PubMed] [Google Scholar]
- A case of tubular apocrine adenoma with syringocystadenoma papilliferum that developed in a nevus sebaceus. Ann Dermatol. 2010;22:319-22.
- [CrossRef] [PubMed] [Google Scholar]
- Syringocystadenoma papilliferum in co-existence with tubular apocrine adenoma on the calf. Ann Dermatol. 2011;23(Suppl 2):S175-8.
- [CrossRef] [PubMed] [Google Scholar]
- An unusual presentation of a congenital benign apocrine hamartoma. J Cutan Pathol. 1990;17:53-8.
- [CrossRef] [PubMed] [Google Scholar]







