Translate this page into:
The effect of topical 0.5% humic acid gel on male rats with skin ulcer
Address for correspondence: Prof. Nematollah Gheibi, Department of Biophysics, Biochemistry and Genetics, Qazvin University of Medical Sciences, Qazvin 34199-15315, Iran. E-mail: gheibi284@yahoo.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Humic derivatives have antibacterial and anti-inflammatory properties.
Aim:
This study aimed to assess the experimental wound-healing effect of 0.5% humic acid gel.
Materials and Methods:
A full-thickness skin wound was created on the dorsal side of 24 Sprague Dawley male rats (220–250 g). The animals were then randomly divided into the control, sham, and experimental groups. Skin wounds were bandaged daily using sterile gauze dipped in normal saline, carboxymethylcellulose, and 0.5% humic acid for 21 days. The wound-healing rate was evaluated grossly and histologically at various time intervals post-injury.
Results:
Wound-healing percentage was significantly higher in the gel treatment group at all time points (P < 0.05). The mean number of inflammatory cells was significantly lower in the humic acid gel group than in the other groups (P < 0.001). Moreover, the number of new vascular cells and fibroblasts were significantly increased in the humic acid gel compared to the control (P < 0.001).
Conclusion:
These data confirmed that 0.5% humic acid gel accelerates wound healing, probably by anti-inflammatory effects, as well as by promoting vascular and fibroblast proliferation. Therefore, the humic acid gel may be used to improve wound care.
Keywords
Healing
humic acid
inflammatory cells
rat
wound
INTRODUCTION
Wounds affect the quality of life, healthcare systems, and increase the cost of hospitalization. Therefore, it makes a lot of sense to find treatments that accelerate wound healing.[12] Wound healing is a dynamic and complex process that replaces the damaged cellular structures and tissue layers. Healing progression is influenced by several factors, such as inflammatory and non-inflammatory cells, mediators, cytokines, extracellular matrix, and vessels.[34]
Several new methods, such as cell and growth factor-based therapy, tissue engineering, and nanotechnology, have recently been introduced. Developed approaches affect the inflammatory, and non-inflammatory phases and cells, cytokines and mediators, extracellular matrix, and angiogenesis; however, these are very expensive and require excessive equipment.[25,6,7,8,9] Recently, public attention to the use of natural substances and traditional therapies has increased, and some mechanisms involved in these substances have been reported.[10,11,12]
Humic derivatives, as nutraceutical substances, have excited physicians since ancient times. Humic substances, including humic acids, fulvic acids, and humin, are harvested during soil decomposition.[13] Humic acid has been shown to have antibacterial, antioxidant, immune-modulating, and anti-inflammatory properties,[1415] which can affect wound healing. There is evidence of the role of homine-containing substances in thrombosis formation and coagulation in the early stages of wound healing. This effect was achieved by increasing the secretion of specific plasminogen activators. In addition, homine-containing substances prevent the formation of fibrin monomers from fibrinogen and thrombus lysis.[16] Sodium humate has been shown to activate the transforming growth factor-β (TGF-β)/Smad signaling pathway in a rat model.[17] It also increases the expression level of TGF-β mRNA and protein 1-3.[18] In addition, humic gel alters the levels of tumor necrosis factor-α (TNF-α) and type III collagen.[19] Humic acid is a more stable compound with stronger pharmacological effects compared with humic sodium. Therefore, it could be a therapeutic target for wound healing.[20] In the present study, the healing properties of gel containing 0.5% humic acid were investigated using an experimental wound model.
Methods and Materials
Animal
Twenty-four Sprague Dawley male rats (230–250 g) were obtained from the Razi Institute (Karaj, Iran). They were individually caged and kept under standard conditions (23 ± 2°C temperature and 55% humidity with 12-h light/dark cycles) in an animal house. The animals were handled in accordance with the Declaration of Helsinki 1975 and the ethical instructions of the Ethics Committee of Qazvin University of Medical Sciences (ethical code: IR.QUMS.REC.1399.220).
Anesthesia and surgery
Anesthesia was induced by intra-peritoneal administration of ketamine-xylazine (50/5 mg/kg; Merck, Darmstadt, Germany), and then the nape of each animal’s neck was shaved and cleaned with Betadine (Tolid Darou, Tehran, Iran). Next, a circular, full-thickness incision (2 cm in diameter) was made using a disposable punch. The rats were kept individually in a clean cage, and the wound site was washed daily with normal saline and bandaged with sterile gauze in all groups.[6] After wound creation, rats were randomly divided into three groups (n = 8): Control, Sham, and treatment groups. In addition, in the sham group, carboxymethylcellulose, and in the treatment group, 0.5% humic acid gel was rubbed topically once a day for 21 days.
Preparation of humic acid gel
A 0.5% humic acid gel was prepared by dissolving 0.004 g of humic acid (Sigma-Aldricha Company, St. Louis, Missouri, USA) and 8 g carboxymethylcellulose (Merck, Germany) in 16 cc of normal saline in a glass beaker. The prepared poultice was stored at 37°C or room temperature until treatment onset.
Healing rate measurement
The rats were placed in a standard crouching position, and the wound area was drawn on transparent paper on days 1, 7, 14, and 21 post-surgery. The ulcer surface was then calculated using AutoCAD software.[8] A photograph of the ulcer area in each rat was also taken. The wound-healing percentage was calculated with the following formula: Wound-healing percentage = Initial wound surface − Nth day wound surface/Initial wound surface × 100
Histomorphologic study
The efficacy of each treatment was also evaluated histologically on days 7, 14, and 21 post-surgery. Skin biopsies (5 µm thick) were obtained from the edges of the wound after induction of anesthesia (50/5 mg/kg). Harvested skin samples were fixed in 10% buffered formalin and blocked with paraffin. Serial sections were prepared using the Leitz microtome. Hematoxylin and eosin staining were used to observe angiogenesis, inflammatory cells, and fibroblasts. Neutrophils, eosinophils, and fibroblasts were counted using a 400× objective lens. To count angiogenesis, first regions with a high density of new vessels were identified using a 100× objective lens, and then three fields in the regions were selected and counting was performed with 400× magnification. An Iwf-Iox-Holland ocular fragment was used for cell counting.[11] Histologically, angiogenesis scoring was performed as shown in Table 1. Toluidine blue staining was used to evaluate and count mast cells.[21]
| Element | Classify | 0 | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Angiogenesis | No evidence of blood vessels | 4–8 vascular channels | 12–15 vascular channels | 15–20 vascular channels | More than 20 vascular channels | |||||
Statistical analysis
The obtained data are expressed as mean ± SEM error of the mean. Data analysis was performed using the SPSS software v.22. The ANOVA statistical method and post-hock Tukey’s test were used; P < 0.05 was assigned significance.
RESULTS
Wound-healing rate
On day 1 posttreatment, the wound-healing rate was zero in all the groups. The humic acid gel significantly improved the wound area compared to the sham and control groups on days 7, 14, and 21 post-surgery (P < 0.001) [Figures 1 and 2]. On day 28 posttreatment, the wound area was completely closed in the humic acid group.
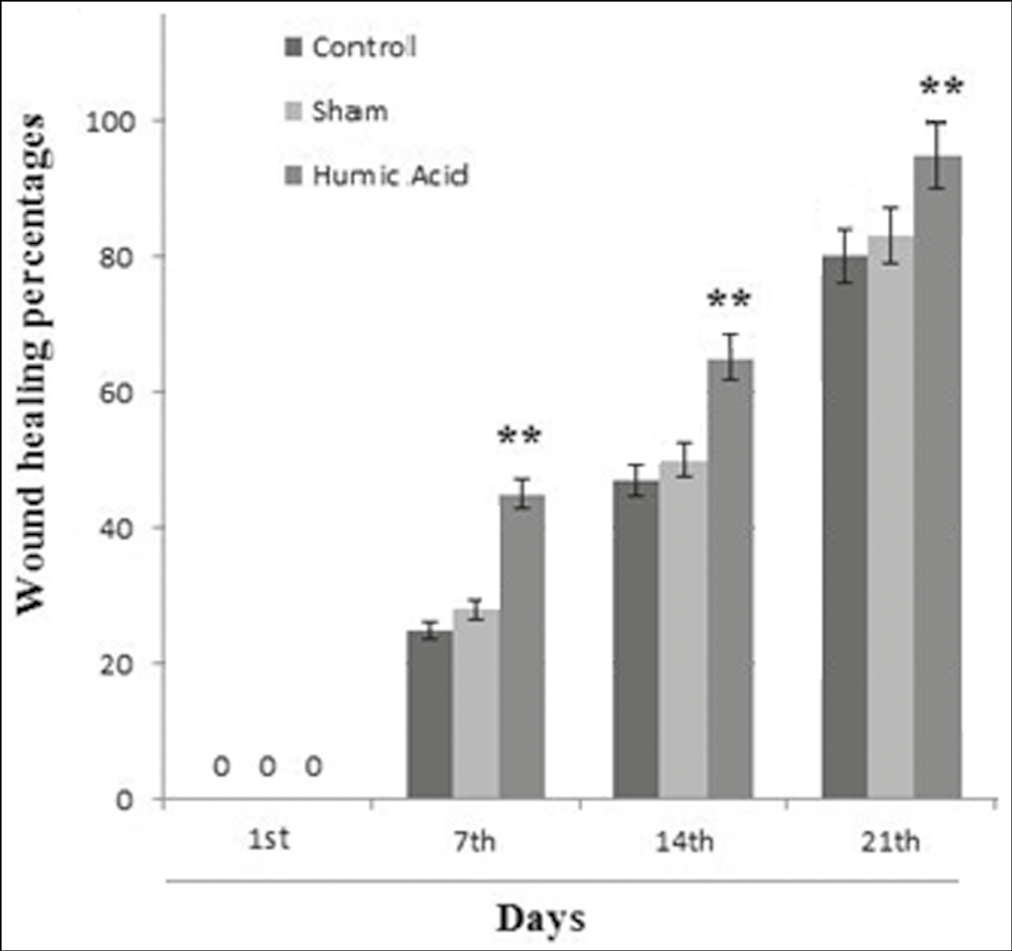
- The percentage of healing in the groups at days 1, 7, 14, and 21 posttreatment (n = 8 rats/group). ** Significant change in comparison with control group P < 0.01
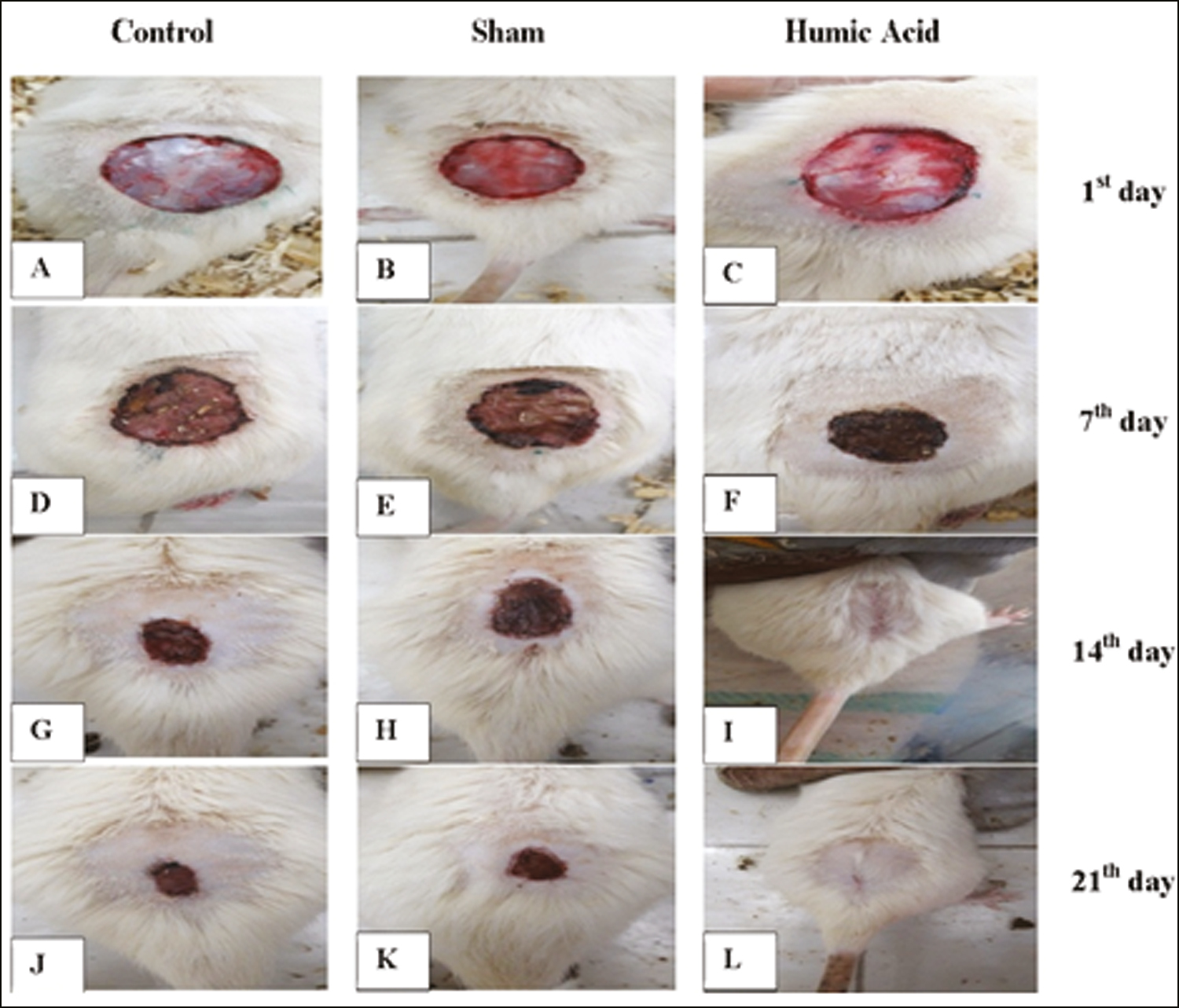
- (A–L): Gross evaluation of wounds healing in all three groups at days 1, 7, 14, 21 post-treatmen. Wound area, control group (A, D, G, J), sham group (B, E, H, K) and humic acid group (C, F, I, L) at days 1, 7, 14, 21 posttreatment, respectively
Inflammatory cells
Microscopic findings showed that there was no significant difference between all groups with respect to the mean percentage of inflammatory cells on day 1 posttreatment (P > 0.05). However, a significant decrease in the mean number of neutrophils, eosinophils, and mast cells was found in the humic acid gel group compared with the control and sham groups on days 7, 14, and 21 post-surgery (P < 0.05) [Table 2]. The mean percentage of these cells was significantly reduced in each group from day 7 posttreatment to the end of the study period [Table 2 and Figure 3A–H].
| Indicator/group | Day | Control | Sham | Humic acid |
|---|---|---|---|---|
| Neutrophils | 1 | 148.0 ± 9.07 | 149.75 ± 9.62 | 146.63 ± 9.62 |
| 7 | 89.75 ± 6.65 | 87.0 ± 3.0 | 64.50 ± 3.93* | |
| 14 | 68.13 ± 5.82 | 60.0 ± 3.5 | 46.00 ± 5.68* | |
| 21 | 49.50 ± 5.32 | 42.1 ± 6.3 | 23.75 ± 5.23* | |
| Eosinophils | 1 | 59.5 ± 4.3 | 60.1 ± 4.3 | 60.1 ± 3.6 |
| 7 | 50.0 ± 3.4 | 44.0 ± 2.3 | 30.2 ± 2.7* | |
| 14 | 38.2 ± 2.8 | 32.8 ± 3.7 | 20.1 ± 3.0* | |
| 21 | 29.0 ± 3.8 | 23.6 ± 3.4 | 9.8 ± 3.2* | |
| Mast cells | 1 | 45.0 ± 3.77 | 44.75 ± 3.37 | 45.0 ± 4.6 |
| 7 | 35.00 ± 2.67 | 31.25 ± 3.24 | 24.8 ± 3.4* | |
| 14 | 27.37 ± 4.10 | 22.12 ± 3.60 | 15.3 ± 3.2* | |
| 21 | 20.00 ± 3.46 | 16.00 ± 3.11 | 10.0 ± 2.6* | |
| Fibroblasts | 1 | 0 | 0 | 0 |
| 7 | 15.3 ± 4.3 | 24.7 ± 3.2 | 45.0 ± 4.5* | |
| 14 | 30.1 ± 2.9 | 37.1 ± 3.6 | 75.0 ± 3.9* | |
| 21 | 75.0 ± 3.2 | 82.0 ± 3.2 | 114.8 ± 2.9* | |
| Angiogenesis | 1 | 0 | 0 | 0 |
| 7 | 7.5 ± 2.3 | 15.0 ± 2.8 | 25.0 ± 4.8* | |
| 14 | 14.1 ± 3.3 | 18.2 ± 2.9 | 44.8 ± 3.2* | |
| 21 | 5.1 ± 1.8 | 7.8 ± 2.4 | 18.0 ± 3.1* |
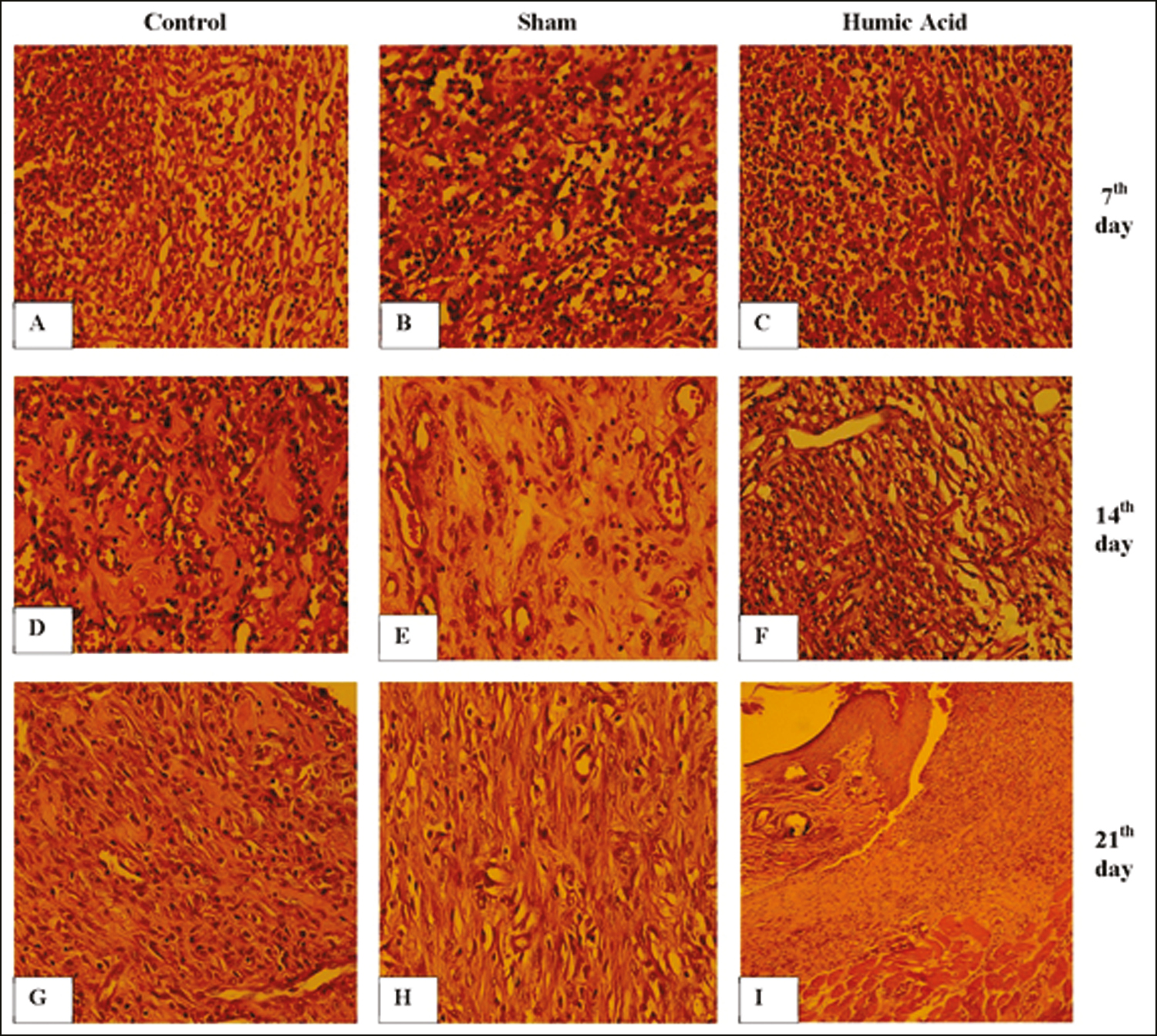
- (A–I) Histomorphologic results of wound healing in all three groups. The infiltration of inflammatory cells, fibroblast proliferation, and angiogenesis in the control (A, D, G), the sham (B, E, H) and the humic acid (C, F, I) at days 7, 14, and 21 posttreatment, respectively. Hematoxylin and eosin staining, 400× and 100× (I)
Fibroblasts
No fibroblasts were detected on day 1 of the study. A significant increase in fibroblasts was observed in the humic acid group compared to the sham and control groups on days 7, 14, and 21 post-surgery (P < 0.05) [Table 2]. The mean percentage of fibroblasts was significantly enhanced in each group from day 7 posttreatment to the end of the study period [Table 2 and Figure 3A–H].
The mean number of vessels
No vessels were detected on day 1 of the study. Treatment with humic acid significantly increased the mean number of new vessels compared to the sham and control groups at 7, 14, and 21 days posttreatment (P < 0.05) [Table 2 and Figure 3A–H].
DISCUSSION
This study evaluated the efficacy of 0.5% humic acid gel in the wound-healing process on days 7, 14, and 21 post-surgery, representing inflammation, proliferation, recovery, and tissue remodeling.[22] Our results showed that 0.5% humic acid spatially accelerated the closure rate of wounds in comparison to the sham and control groups from the second to the fourth-week post-surgery.
This finding is in accordance with those reported by Çalişir et al.[15]; however, they administered humic acid at different concentrations for a 3-mm full-thickness wound versus a 2-mm area in the present study. In addition, the wound-healing effects of humic acid have been reported by Ji et al.[17] They used sodium humate at a concentration of 1%, associated with fibroblast growth factor.
The findings of the present study confirm the anti-inflammatory effects of humic acid. The 0.5% gel of humic acid significantly reduced the proliferation of inflammatory cells, including neutrophils, eosinophils, and mast cells, in the early phase of the wound-healing period, which can accelerate wound healing. Microscopic findings also confirmed the proliferation of fibroblasts and new vessels in the treatment group in which the wounds were completely closed at the end of the study in the humic group.
The wound-healing process is dynamic and complex. It is involved in cell proliferation, control of the inflammatory process, and reepithelization. The regulation of secreted cytokines reduces the infiltration of inflammatory cells, such as neutrophils and eosinophils, leading to an increase in cellular proliferation.[23] In this regard, Junek et al.[24] showed that humic acid induces cytokine release, such as TNF-α, from differentiated U937 cells via the inhibition of 5-lipoxygenase. These cytokines are released from neutrophilic granules. Yalman and Laçin[19] demonstrated that the humic substances are effective on the immune system through the inhibition of pro-inflammatory mediators and increase of TNF-α. Moreover, Spilioti et al.[25] revealed that humic acid reduced the release of inflammatory factors. Humic acid also suppresses the lipoxygenase pathway that induces leukotriene formation.[26] On the other hand, humic acid substances can increase granules containing collagen and fibroblasts, which are important in the remodeling phase. This suggests that humic acid upregulates growth factors, such as TGF-β, which play an important role in the late phase of wound healing.[17]
The effect of 0.5% humic acid gel on the proliferation of fibroblasts and neovascularization was the main finding of our study. These findings showed that humic acid also improved wound healing through its effect on the proliferation phase. The formed granulation tissue is mostly fibroblasts, and angiogenesis occurs in the proliferative phase of the wound-healing process.[27] The remodeling phase is characterized by the reformation and improvement of collagen fiber components.[3] Similar findings have shown that hydrogels containing humic acid decrease the expression level of type I collagen and increase that of type III collagen.[19] Overall, most of the recent studies are consistent with the results of our study and report the benefit of humic substances on wound-healing improvement.
CONCLUSION
The present study demonstrated that 0.5% humic acid gel accelerated wound healing in male rats. This effect may be due to the anti-infiltratory and stimulatory effects of the humic acid gel on fibroblasts and vascular promotion. Therefore, this gel may be useful for the treatment of skin wounds.
Authors contributions
FS, MS, NG: The study concept and design, data collection, or analysis and interpretation of data. FS, NG, FG, EB: Writing of the manuscript or critical review of important intellectual content, critical review of the literature. ZS, AT: Methodology. All authors: Final approval of the final version of the manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank the Research Center of Qazvin University of Medical Sciences.
REFERENCES
- Critical-sized full-thickness skin defect regeneration using ovine small intestinal submucosa with or without mesenchymal stem cells in rat model. J Biomed Mater Res B Appl Biomater. 2018;106:2177-90.
- [Google Scholar]
- Embryonic stem cell extracts improve wound healing in diabetic mice. Acta Diabetol. 2020;57:883-90.
- [Google Scholar]
- Skin wound healing: An update on the current knowledge and concepts. Eur Surg Res. 2017;58:81-94.
- [Google Scholar]
- Effect of acacia honey-impregnated placenta membrane on pain and burn wound repair. Comp Clin Pathol. 2018;27:1457-63.
- [Google Scholar]
- Effects of the oral administration of silver nanoparticles on wound healing in male rats. Wound Practice Res. 2020;28:8-16.
- [Google Scholar]
- The effect of oral consumption of propolis alone and in combination with silver nanoparticles on wound healing in male Wistar rats. Wound Manag Prev. 2020;66:38-46.
- [Google Scholar]
- Chronic wound healing: A review of current management and treatments.Adv Ther. . 2017;34:599-610.
- [Google Scholar]
- The effect of honey-impregnated human placenta membrane on burn wound healing in rat. Comp Clin Path. 2015;24:263-8.
- [Google Scholar]
- Royal jelly accelerates healing of acetate induced gastric ulcers in male rats. Gastroenterol Hepatol Bed Bench. 2020;13:14-22.
- [Google Scholar]
- Tau oligomers as potential targets for Alzheimer’s diagnosis and novel drugs. Front Neurol. 2013;4:167.
- [Google Scholar]
- Evaluation of healing effects of poultice containing 05% fulvic acid on male white-male rats with skin ulcer. J Cutan Aesthet Surg. 2022;15:40-7.
- [Google Scholar]
- Nutraceuticals’ novel formulations: The good, the bad, the unknown and patents involved. Recent Pat Drug Deliv Formul. 2019;13:105-56.
- [Google Scholar]
- Humic acid enhances wound healing in the rat palate. Evidence-based Complement Alternat Med. 2018;2018:1783513.
- [Google Scholar]
- Humic substances as a potent biomaterials for therapeutic and drug delivery system—a review. Int J Appl Pharm. 2019;11:1-4.
- [Google Scholar]
- Sodium humate accelerates cutaneous wound healing by activating TGF-β/Smads signaling pathway in rats. Acta Pharm Sin B. 2016;6: 132-40.
- [Google Scholar]
- Evaluation of wound healing activity of sodium humate on rat. Chin J Dermatol. 2012;9: 793-6.
- [Google Scholar]
- Development of humic acid and alginate-based wound dressing and evaluation on inflammation. Mater Technol. 2019;34:705-17.
- [Google Scholar]
- A comparison of the compositional differences between humic fractions isolated by the IHSS and exhaustive extraction procedures. Naturwissenschaften. 2014;101:197-209.
- [Google Scholar]
- Topical estrogen accelerates wound healing in diabetic rats. Physiol Pharmacol. 2011;242:242-251.
- [Google Scholar]
- Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J Tissue Viability. 2016;25:98-118.
- [Google Scholar]
- Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res Ther. 2020;11:232.
- [Google Scholar]
- Bimodal effect of humic acids on the LPS-induced TNF-α release from differentiated U937 cells. Phytomedicine. 2009;16:470-6.
- [Google Scholar]
- Biological properties of mud extracts derived from various spa resorts. Environ Geochem Health. 2017;39:821-33.
- [Google Scholar]
- The anti-inflammatory effects of lipoxygenase and cyclo-oxygenase inhibitors in inflammation-induced human fetal glia cells and the Aβ degradation capacity of human fetal astrocytes in an ex vivo assay. Front Neurosci. 2017;11:299.
- [Google Scholar]
- The effects of vitamin e and selenium on blood flow to experimental skin burns in rats using the 133Xe clearance technique. Open Med. 2010;5:219-23.
- [Google Scholar]






