Translate this page into:
Therapeutic Efficacy of a Plasma Rich in Growth Factors Gel Combined with Fractional Ablative Laser in the Management of Post-acne Scars
Address for correspondence: Dr. Eduardo Anitua, Eduardo Anitua Foundation, Jacinto Quincoces 39, Vitoria, Spain. E-mail: eduardo@fundacioneduardoanitua.org
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Acne vulgaris is a common condition that often results in secondary cutaneous damage in the form of scarring. Scars require shape-specific scaffolds. Recently, a new 3D gel derived from plasma rich in growth factors technology (PRGF) has been developed with the aim of overcoming these limitations.
Objective:
The aim of this study was to preliminarily assess the clinical performance of the combination therapy with PRGF-gel (PG) and fractional ablative laser for post-acne scar amelioration.
Materials and Methods:
Nine patients suffering from post-acne scars received a combination of PG and fractional ablative laser therapy. Macrophotographs were taken and patients completed a satisfaction survey. Images were also analyzed following the ECCA score. Clinicians were also asked to fulfill a clinical improvement score and any undesired side effects were recorded.
Results:
Patients were referred to be highly satisfied as an 8.7 ± 0.9 satisfaction score was achieved. Healthcare specialists objectivated that the scar reduction and overall skin quality at the end of the study had noticeably improved. The ECCA score showed a significant 55% of improvement compared with baseline. No major side effects were recorded, and the tolerance of the treatment was excellent.
Conclusion:
The combined therapy with PG and fractional ablative laser might help in the management of post-acne scars and overall skin rejuvenation.
Keywords
Ablative laser
acne scars
growth factors
platelet-rich plasma
protein gel
INTRODUCTION
Acne vulgaris is a common condition that affects 80% of teenagers and in some cases it remains to the adulthood with a main psychosocial effect.[1] It is caused and characterized by multiple factors including Propionibacterium acnes activity, androgenic stimulation, or increased sebum production.[2] Inflammatory acne lesions often result in secondary cutaneous damage in the form of scarring which is defined as fibrous tissue that replaces normal skin that is destroyed by injury or disease.
The information about acne scarring pathogenesis is little. Scars normally develop when the optimal wound healing phases of the skin do not reach the required efficacy and the inflammation of the underlying tissue exceeds the normal period. Accordingly, the damaged tissue never reaches the same level of functional and biomechanical performance as the original skin.[3] Some studies have reported that the cell activity and the number of Langerhans and CD4+ T-cells are significantly reduced during acne scar development. Additionally, B-cell infiltration and sebaceous gland structure alteration have also been associated with acne scarring.[4] This is suggestive of an unsuccessful response to the causal antigens that is accompanied by an altered influx of macrophages and skin homing memory and effector cells.[5] These factors are related to collagen and connective tissue damage, which can lead to permanent skin texture changes and fibrosis. Hence, post-acne scars are morphogenetic alterations of the skin that usually provoke functional impairment and aesthetic and psychologic sequelae. In fact, patients suffering from acne scarring develop serious emotional debilitation and anxiety that impairs life quality.[6] Thus, the scarring that results from acne tissue damage and inflammation is a significant issue that requires special attention.
The treatment of acne scars is complex and often requires multiple modalities. Prevention is the optimal method; however, patients do not always present to physicians for prompt diagnosis and the delay in treatment increases the probability of secondary acne sequelae as scarring.[7] Hence, different approaches have been described with the aim of reducing post-acne-derived cutaneous fibrosis. Ablative lasers, fractional laser therapy, autologous fat transfer, dermabrasion, chemical peels, radiofrequency, microneedling, or injectable fillers are the primary tools in the modern armamentarium for scars.[8] However, in some cases, these therapeutic options have proved limited efficacy and problematic side effects because the final success depends on the patient’s pathological condition and the severity of the lesions.[9] Hence, new custom-tailored treatments with optimal safety profile and therapeutic efficacy are demanded by clinicians.
In this line, several studies have investigated the role of platelet-rich plasma (PRP) as an assisted therapy for post-acne scars. This treatment is usually based on the extraction of a limited blood volume to obtain a liquid plasma, which is intradermally injected as a monotherapy or in combination with other techniques like ablative lasers.[10] Commercially available PRP-derived products such as PRGF or i-PRF have proved to be easy to prepare and cost-effective.[111213] However, although current results support its regenerative potential, PRP-derived formulations are restricted to their liquid or clot nature, which provide a limited volume enhancer effect. Sometimes, cutaneous defects like scars require shape-specific scaffolds that maintain their structural stability and mechanical integrity for long periods of time. Additionally, the natural fibrin retraction process of PRP and its relatively rapid degradation are a disadvantage when managing volumetric soft tissue increase.
Recently, a new biomaterial derived from plasma rich in growth factors technology (Endoret®PRGF®) has been developed with the aim of overcoming the limitations of current PRP formulations. PRGF-gel (PG) is an injectable scaffold based on the patient’s own proteins that closely resembles the 3D characteristics and biomechanics of native skin.[14] This formulation is 100% autologous and has a gel-like structure that has proved its efficacy and rheological stiffness for its application in cutaneous viscosupplementation.[15] Furthermore, depending on the plasmatic protein denaturation degree, it can be customized into low or high viscosity gel forms to meet different dermatological requirements.[16] Previous studies have biomechanically characterized both gel forms revealing optimal properties to overcome different skin irregularity requirements.[16] The aim of this study was to preliminarily assess the clinical performance of the combination therapy with PG and fractional ablative laser in the management of post-acne scars.
MATERIALS and METHODS
The study was conducted following the principles established in the Declaration of Helsinki amended in 2013, and patients gave their informed consent. All described procedures were performed according to the common clinical practice of the center, and patients gave their informed consent for photograph publication.
Platelet-gel preparation
Manufacturer’s instructions were followed for high viscosity and low viscosity PG preparation, as described previously (KMU10-Gel, BTI Biotechnology Institute, Vitoria, Spain).[14] Eighteen milliliters of blood were collected into 9 mL tubes containing 3.8% (wt./vol.) sodium citrate as anticoagulant and centrifuged for PRP separation. Once the platelet-rich and leucocyte-free fraction was obtained, part of the plasma was thermally gelated. Longer gelation incubations were used for high viscosity PG preparation, whereas shorter incubations were needed for low viscosity PG obtention. The remaining volume was activated with calcium chloride and steadily mixed with the protein gel prior to injection. Slight mixture protocol was used for high viscosity PG preparation, whereas vigorous mixing was applied for low viscosity PG obtention [Figure 1].
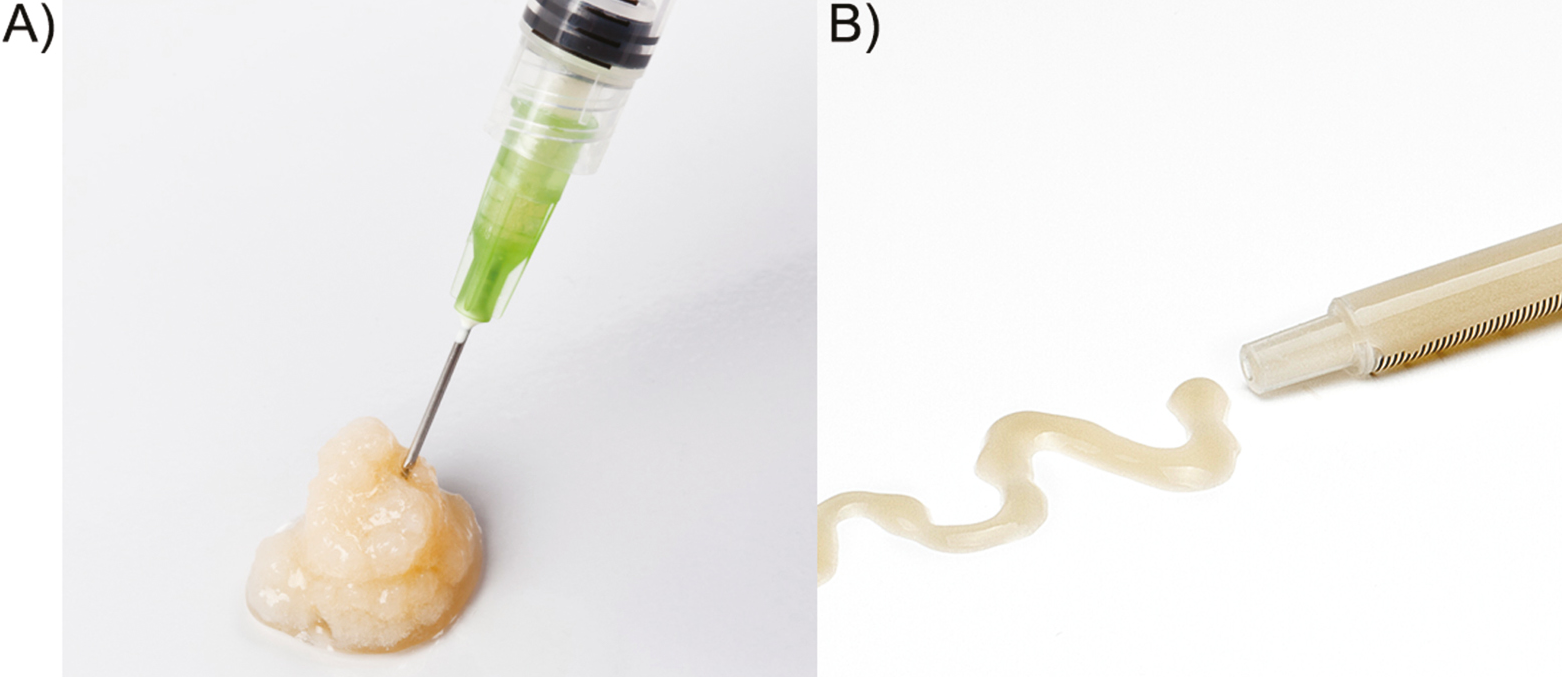
- (A) High viscosity PRGF-Gel. (B) Low viscosity PRGF-Gel
Patients
Following a retrospective design, nine patients between 25 and 50 years old from “Grupo Dermatología Pedro Jaén Clinic” (Madrid, Spain) were included in the study. As inclusion criteria, patients with mild-to-severe post-acne scarring that have been treated with the combined therapy were selected. Patient’s scars have not been previously treated with any treatment modality. Patient’s precise clinical data were collected in their medical records.
Combined therapy
Patients were treated with a combination of PG and fractional ablative laser therapy. All treatments were performed after the application of topical anesthesia. First, varying volumes of high/low viscosity PG were applied following individual requirements to meet the cutaneous topography. The high viscosity gel was used in deep dermal layers of the skin using a 27G cannula. The low viscosity gel was placed more superficially with a scraping technique into the superficial tissue using 30G microneedles. The product was injected around the margins of the lesion and into the scar bed. Afterwards, the patients underwent fractional laser resurfacing with either CO2 laser or erbium YAG laser. The 10,600 nm-CO2 laser (SmartXide DOT, Deka, Calenzano, Italy) was adjusted to 20–30 W, 5–15% density, and 500–1000 ms of dwell time. More aggressive treatments with higher power were used over severe scarring, whereas milder settings were applied in the surrounding areas for each lesion. Similar settings were used with the erbium YAG laser adjusted to 9 × 9 mm spot (Icon 2940, Cynosure, Madrid, Spain). Laser aftercare included daily topical antibiotic for 1 week and strict solar protection ointment until re-epithelization. Patients received one to four sessions of the combined treatment with intervals of 4–6 weeks depending on their clinical evolution. Patient demographics and each combined treatment description are summarized in Table 1.
| Patient | Gender | Age (years) | Sessions | PRGF-gel treatment | Laser treatment |
|---|---|---|---|---|---|
| 1 | Female | 48 | 2 sessions | High/low viscosity (6 mL/3 mL) | Fractional CO2 laser |
| 2 | Male | 39 | 2 sessions | High/low viscosity (6 mL/3 mL) | Fractional CO2 laser |
| 3 | Female | 36 | 3 sessions | Low viscosity (3 mL) | Fractional CO2 laser |
| 4 | Male | 29 | 2 sessions | Low viscosity (3 mL) | Fractional CO2 laser |
| 5 | Female | 35 | 2 sessions | Low viscosity (3 mL) | Fractional CO2 laser |
| 6 | Female | 27 | 1 session | Low viscosity (3 mL) | Fractional CO2 laser |
| 7 | Male | 25 | 2 sessions | Low viscosity (6 mL) | Erbium YAG laser |
| 8 | Female | 32 | 2 sessions | Low viscosity (6 mL) | Erbium YAG laser |
| 9 | Female | 50 | 4 sessions | Low viscosity (6 mL) | Erbium YAG laser |
Clinical assessment
Standardized macrophotographs were taken at baseline and at the end of the treatment. Patient images were analyzed by two trained and blinded clinicians following the ECCA score as a validated scale for acne scar severity grading. Additionally, the clinicians were asked to fulfill a clinical improvement score from 0 (the condition has worsened) to 10 (an intense improvement is observed). Subjects were also asked to complete a self-assessment questionnaire and rated their overall satisfaction following a Likert’s scale from 0 to 10 (where 0 is extremely dissatisfied and 10 is extremely satisfied). Any undesired side effects or adverse reactions were recorded.
RESULTS
Six women and three men were treated with the combined therapy. The mean age of the patients was 36 ± 8 years. Subjects presented Fitzpatrick phototype I–III and suffered from moderate-to-severe post-acne scars. Six participants received fractional CO2 laser, whereas three received erbium YAG laser. The mean session number was 2.2 ± 0.8. Further than the expected recovery period after ablative laser, no additional adverse reactions were recorded. Patient recovery included mild crusting, erythema, and edema for 4–7 days with mild sensation of itching. Post-inflammatory hyperpigmentation or other long-term side effects were not recorded, and the tolerance of the treatment was excellent. At the end of the follow-up period, patients referred to be highly satisfied as an 8.7 ± 0.9 satisfaction score was achieved following the 10-point Likert’s scale [Figure 2(A)]. Healthcare specialists objectivated that the scar reduction and the overall skin quality at the end of the study had noticeably improved (7.4 ± 0.7 score) [Figure 2(A)]. The validated ECCA score highlights the scar severity. Baseline ECCA score was 157 ± 61 and at the end of the study it was significantly lowered to 70 ± 33 (P < 0.05). This revealed an improvement of 55% compared with baseline [Figure 2(B)]. The synergy of laser resurfacing and intradermal gel deposition promoted an early volumetric disposal that was translated into an immediate soft tissue augmentation and scar amelioration effect [Figures 3 and 4]. None of the patients referred to be dissatisfied with the therapy.
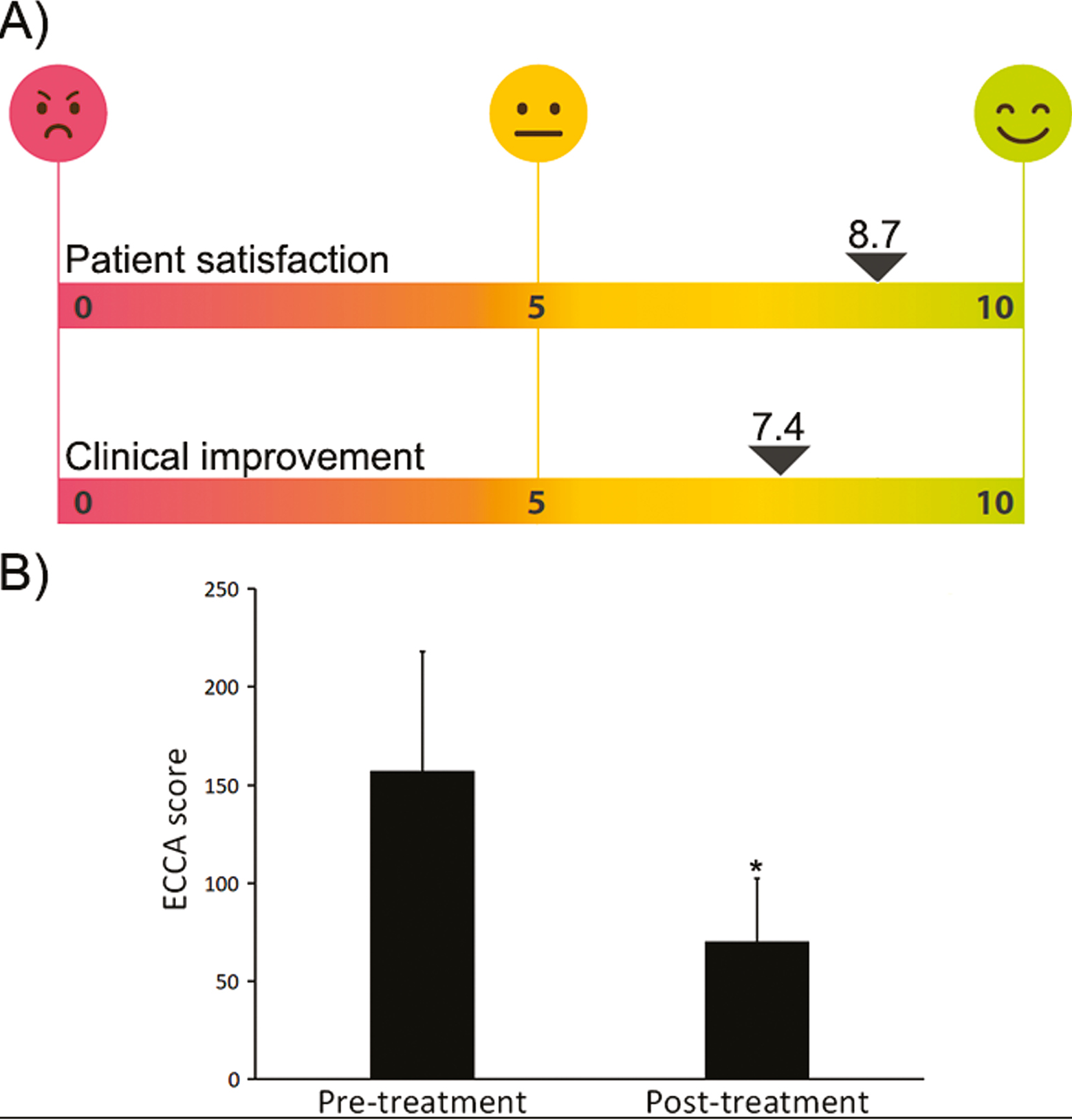
- (A) Self-assessment questionnaires showed that patients referred to be very satisfied with the combined therapy. Healthcare specialists objectivated that the scar reduction and the overall skin quality at the end of the study had noticeably improved. (B) The ECCA score during the study showed a statistically significant reduction which is related to an improvement in the scar severity (*P < 0.05)
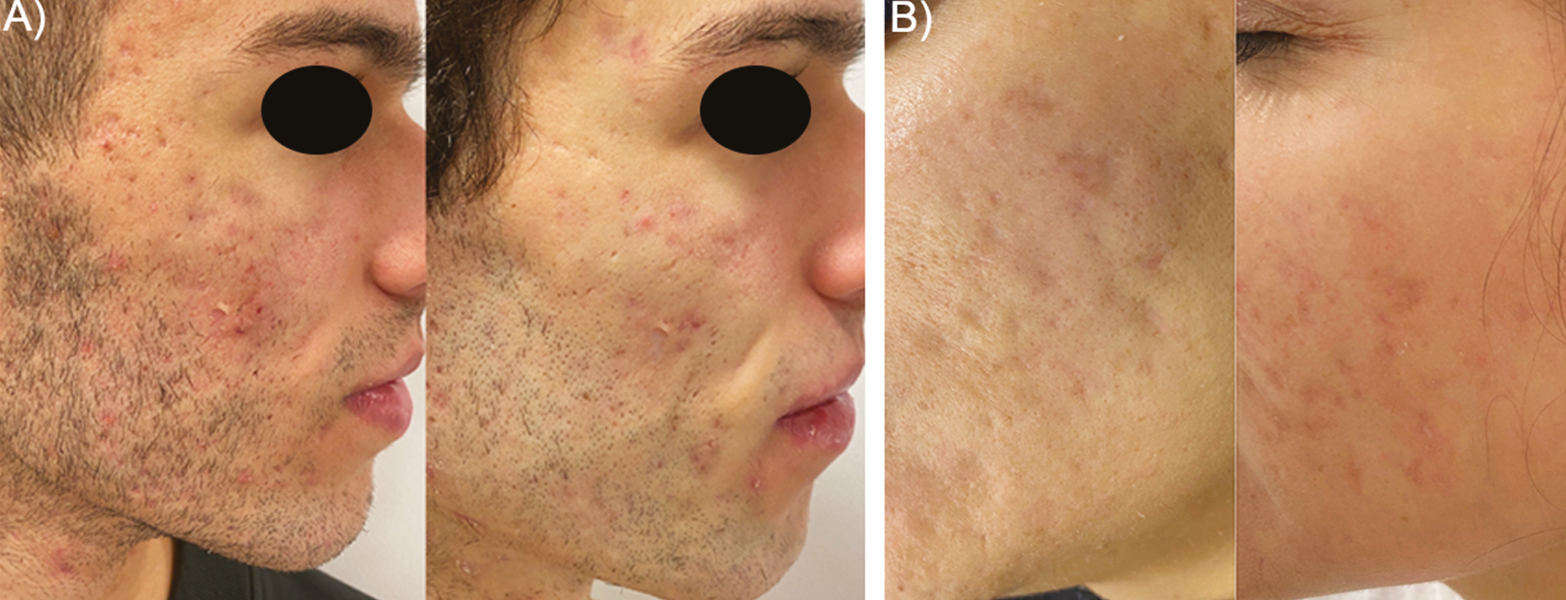
- Representative macrophotographs of two patients (A, B) suffering from post-acne scars. Before (left) and after combined therapy situations (right) show a noticeable clinical improvement of the skin quality and overall scar amelioration
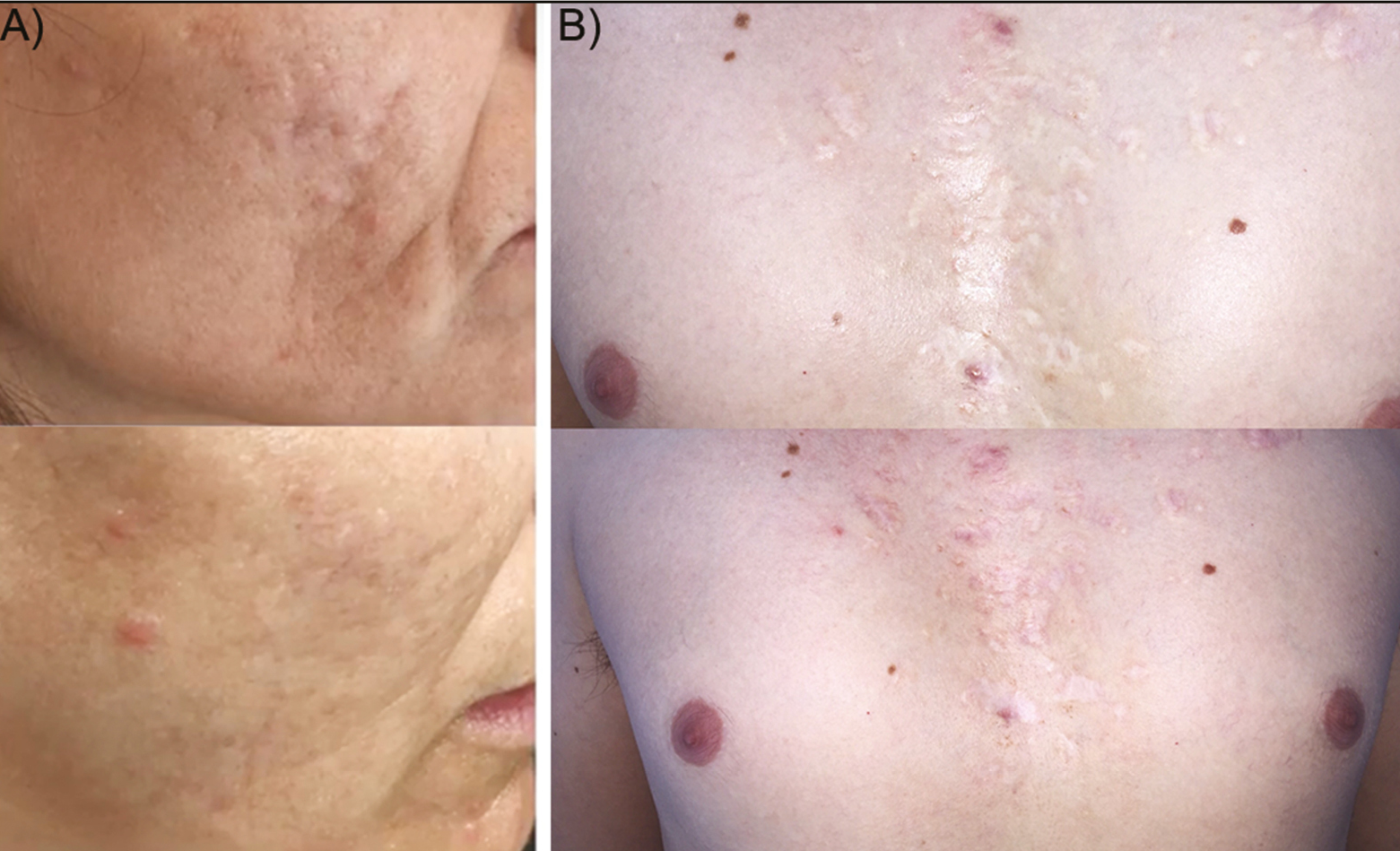
- Representative macrophotographs of two patients (A, B) suffering from post-acne scars. Before (up) and after combined therapy situations (down) show a noticeable clinical improvement of the skin quality and overall scar amelioration
DISCUSSION
Ablative laser therapy is based on the concept of selective photothermolysis, which was first described in 1983 and noted that “selective brief pulses of selectively absorbed optical radiation can cause selective damage to pigmented structures, cells, and organelles.”[17] The dermal heating below the zone of ablation and the healthy tissue surrounding the treated area induce a wound healing response, which causes collagen remodeling and cutaneous contraction.[18]
In this study, ablative CO2 and erbium YAG lasers were used in their fractional versions. The first causes rapid heating and vaporization of tissue at a depth of 20–60 µm as the laser is absorbed by intracellular and extracellular water at a deep dermal layer.[19] The second is more aqueous selective due to its shorter wavelength and vaporizes tissue at a depth of 20–25 µm. The energy is absorbed in the epidermis and papillary dermis, and the laser has a more superficial ablation profile.[20] However, both techniques provoke a recovery period that takes up to 10 days and side effects such as pain, erythema, dyschromia, edema, infection, and demarcation lines may occur.[9] In this sense, PRP has been extensively reported as an optimal adjuvant for ablative laser therapy due to the regenerative potential of the growth factors and bioactive proteins that are released at the site of injection.[21] In fact, several studies have demonstrated that autologous molecules derived from the patient’s own blood improve the quality of post-scar tissue and decrease the duration and severity of laser-related side effects.[22] However, post-acne scars usually comprise significant cutaneous defects that need deep dermal augmentation and volume restitution. Common PRPs are liquid formulations that provide a boost of cytokines and signaling molecules, but they have short half-life and provide low filler effect. Hence, they do not meet shape-specific and three-dimensional requirements for prolonged periods of time.
In this study, a novel formulation derived from plasma rich in growth factors technology (Endoret®PRGF®) has been used as an autologous long-lasting gel for cutaneous projection in combination with ablative laser therapy. PRGF is a specific type of PRP that allows the safe and versatile modification of plasmatic physicochemical properties with the aim of designing custom-tailored bioproducts that meet specific clinical needs.[23] Other formulations derived from PRGF such as eye drops, topical serums, or fibrin clots have been already evaluated in different medical fields including ophthalmology, oral surgery, traumatology, and dermatology.[24252627] Previous rheological characterization of PG shows that both high and low viscosity PG has a viscoelastic behavior with optimal cohesivity that allows a precise injection while keeping a localized distribution once extruded. While high viscosity PG shows a higher stiffness profile, low viscosity PG presents a moderate pseudoplastic nature.[16] In fact, recent reports demonstrate that the combination of PG and PRGF improves the final clinical outcome compared with the use of PRGF alone when managing skin rejuvenation.[28]
Results presented herein demonstrated that the combination therapy of PG and ablative laser can reduce the severity of post-acne scars in a safe and effective way. Patients referred a high satisfaction at the end of their treatments, and clinicians objectivated a noticeable skin quality improvement. The synergy of laser resurfacing and intradermal gel deposition promoted an early volumetric disposal that was translated into an immediate soft tissue augmentation and scar amelioration effect. This could be related to the strong biomechanical performance of PG that allows the gel to behave as a cohesive scaffold once implanted in the living tissue rather than spreading out to the surrounding microenvironment.[14] These results are consistent with previous reports that support the use of PG in atrophic scars derived from acute trauma.[29] Moreover, skin quality improvement and soft tissue restitution have been also demonstrated after PG monotherapy when managing moderate-to-severe wrinkle amelioration and skin rejuvenation.[1628,30]
It is also noteworthy to consider the high load of growth factors that PG releases to the injured skin once the photothermolysis occurs.[14] These morphogens have proved to promote surrounding dermal fibroblast chemotaxis and proliferation into the porous surface of PG, which ultimately synthetize extracellular matrix proteins such as collagen and hyaluronic acid in a three-dimensional arrangement.[14] Therefore, PG may act as a transient scaffold for cellular recruitment and ingrowth that might accelerate the skin regeneration after aggressive treatments such as ablative lasers.
This study has some limitations including the retrospective design and the low patient number. Patient personalized therapy followed individual requirements, so the results are not based on uniform treatment regimens. Hence, additional prospective studies including randomized and controlled trials should be performed to evaluate the efficacy and safety of the combination of PG and ablative lasers. However, the preliminary findings reported in the present study suggest that this combined therapy could help in the management of post-acne scars and overall skin rejuvenation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
Eduardo Anitua is the scientific director and Ander Pino is researcher at BTI Biotechnology Institute, the company that has developed the Endoret®PRGF® technology. The rest of the authors declare no conflict of interest.
Contribution details
PJ contributed to the manuscript review. EA contributed to the intellectual content. AP contributed to the design and manuscript preparation. AA and NJ contributed to clinical study and data aquisition.
REFERENCES
- Practical evaluation and management of atrophic acne scars: Tips for the general dermatologist. J Clin Aesthet Dermatol. 2011;4:50-7.
- [Google Scholar]
- Effective treatments of atrophic acne scars. J Clin Aesthet Dermatol. 2015;8:33-40.
- [Google Scholar]
- Atrophic scar formation in patients with acne involves long-acting immune responses with plasma cells and alteration of sebaceous glands. Br J Dermatol. 2018;179:906-17.
- [Google Scholar]
- The role of innate immunity in the pathogenesis of acne. Dermatology. 2003;206:96-105.
- [Google Scholar]
- The psychosocial impact of acne: Patients’ perceptions. J Am Acad Dermatol. 1995;32:S26-30.
- [Google Scholar]
- Evaluating evidence for atrophic scarring treatment modalities. JRSM Open. 2014;5:2054270414540139.
- [Google Scholar]
- The treatment of acne scars, a 30-year journey. Am J Clin Dermatol. 2019;20:683-90.
- [Google Scholar]
- A meta-analysis of the evidence for assisted therapy with platelet-rich plasma for atrophic acne scars. Aesthetic Plast Surg. 2019;43:1615-23.
- [Google Scholar]
- Cost-effectiveness of platelet-rich plasma for diabetic foot ulcer in spain. Int J Low Extrem Wounds. 2021;20:119-27.
- [Google Scholar]
- Clinical and economic effectiveness of the use of platelet-rich plasma in the treatment of chronic wounds. Wound Med. 2017;19:27-32.
- [Google Scholar]
- Effectiveness and efficiency of platelet rich plasma in the treatment of diabetic ulcers. Curr Pharm Biotechnol. 2015;16:630-4.
- [Google Scholar]
- A novel personalized 3D injectable protein scaffold for regenerative medicine. J Mater Sci Mater Med. 2017;29:7.
- [Google Scholar]
- Plasma rich in growth factor gel as an autologous filler for facial volume restoration. J Cosmet Dermatol. 2020;19:2552-9.
- [Google Scholar]
- Autologous platelet-rich gel for facial rejuvenation and wrinkle amelioration: A pilot study. J Cosmet Dermatol. 2018;18:1353-60.
- [Google Scholar]
- Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-7.
- [Google Scholar]
- Wrinkles and acne scars: Ablative and nonablative facial resurfacing. In: Raulin C, Karsay S, eds. Laser and IPL Technology in Dermatology and Aesthetic Medicine. Berlin: Springer; 2011. p. :289-97.
- [Google Scholar]
- Treatment of atrophic facial acne scars with a dual-mode er:YAG laser. Dermatol Surg. 2002;28:551-5.
- [Google Scholar]
- A meta-analysis of fractional CO2 laser combined with PRP in the treatment of acne scar. Lasers Med Sci. 2020;36:1-12.
- [Google Scholar]
- Efficacy of autologous platelet-rich plasma combined with ablative fractional carbon dioxide laser for acne scars: A systematic review and meta-analysis. Aesthet Surg J. 2019;39:NP279-87.
- [Google Scholar]
- Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J Control Release. 2012;157:29-38.
- [Google Scholar]
- A randomized clinical trial evaluating plasma rich in growth factors (PRGF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28:1070-8.
- [Google Scholar]
- Autologous serum and plasma rich in growth factors in ophthalmology: Preclinical and clinical studies. Acta Ophthalmol. 2015;93:e605-14.
- [Google Scholar]
- Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529-35.
- [Google Scholar]
- Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater. 2008;84:415-21.
- [Google Scholar]
- Combined therapy with Endoret-Gel and plasma rich in growth factors vs Endoret-Gel alone in the management of facial rejuvenation: A comparative study. J Cosmet Dermatol. 2020;19:2616-26.
- [Google Scholar]
- In vitro characterization and clinical use of platelet-rich plasma-derived Endoret-Gel as an autologous treatment for atrophic scars. J Cosmet Dermatol. 2020;19:1607-13.
- [Google Scholar]
- An autologous protein gel for soft tissue augmentation: In vitro characterization and clinical evaluation. J Cosmet Dermatol. 2019;18:762-72.
- [Google Scholar]






