Translate this page into:
Treatment of Laser Resistant Granuloma Faciale with Intralesional Triamcinolone acetonide and 5-Fluorouracil Combination Therapy
Address for correspondence: Dr. Diana L Norris, C/O RMH, Grattan St, Parkville, Victoria - 3204, Australia. E-mail: diana_mercuri@yahoo.com.au
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This report describes a sixty year old male with biopsy proven Granuloma Faciale (GF). The patient had been unsuccessfully treated with multiple therapies. A mixture 0.8 ml 5-Fluorouracil (5FU) and 0.2 ml Kenacort-A was trialled initially to treat this patient, followed by a more varied mixture ratio. These were given at intervals ranging from two weeks to two months. The patient received a total of twenty injections over a period of more than three years. An excellent response was noted and the patient is now able to tolerate long treatment free periods of between nine and twelve months. 5FU is a simple injection material and can be considered by clinicians as an option for treatment of GF.
Keywords
Combination therapy
granuloma faciale
intralesional steroid
5-fluorouracil
INTRODUCTION
Granuloma faciale (GF) is an uncommon benign inflammatory condition of the skin with characteristic clinical and histological features. Pinkus originally described the name in 1952, although there had been earlier reports of eosinophilic granulomas.[1] It mostly affects the face, particularly sun-exposed areas such as the cheeks, forehead, nose, preauricular region and ears. Patients are most commonly middle-aged white males, and affected sites are often asymptomatic. The clinical differential diagnosis includes lupus vulgaris, discoid lupus erythematosus, fungal infection, sarcoidosis, mycobacteriosis, lymphocytoma cutis, Jessner's lymphocytic infiltrate and cutaneous lymphoma.[2] Histopathology results show a dense granulomatous infiltrate of the upper two-thirds of the dermis, often with associated eosinophils.[3] The epidermis is spared, with a Grenz zone and leucocytoclastic vasculitis in the upper dermis.
CASE REPORT
A man in his sixties was referred for evaluation of biopsy proven GF [Figure 1]. He had a known history of epilepsy. The lesion was first noted 15 years prior when a persistent, erythematous, indurated plaque appeared with associated telangiectasia over the right side of his forehead. Histopathologic findings were consistent with granuloma faciale showing a dense mixed infiltrate within the dermis, comprising of plasma cells, eosinophils, neutrophils and macrophages. Findings were consistent with a vasculitis, without fibrinoid necrosis. No defined Grenz zone was noted [Figures 2 and 3].

- Patient prior to treatment
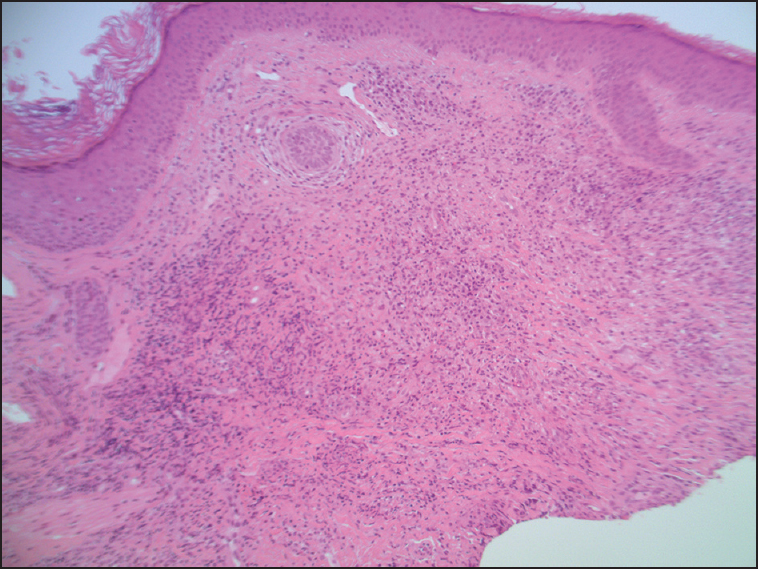
- Histopathology of biopsy; mention magnification power
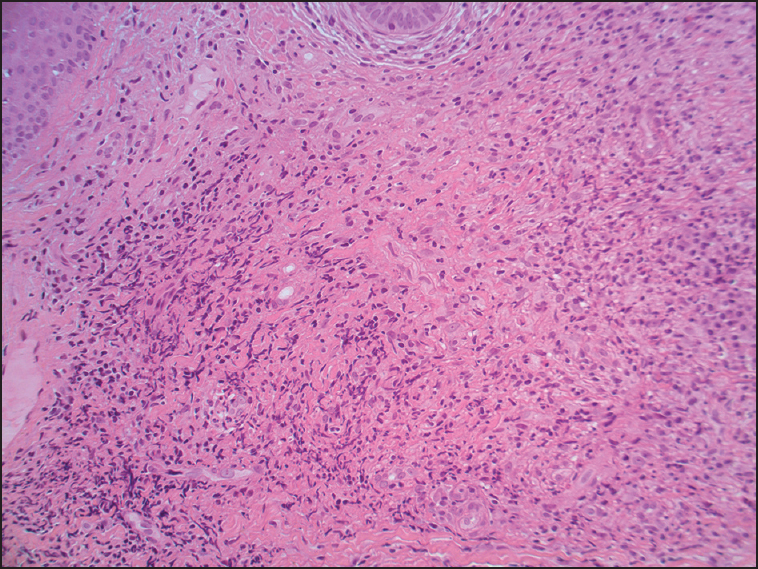
- Histopathology of biopsy; high power mention magnification
GF is notoriously difficult to treat. Multiple modalities have been used in the past with variable success including single therapy or combinations of colchicine, dapsone, antimalarials, gold injections, isoniazid, clofazimine, topical psoralen with UVA, corticosteroids, cryosurgery and various laser therapies.[24] This patient had been treated with a number of these topical therapies prior to referral to our centre, each with poor results.
The patient was initially commenced on topical calcineurin antagonist (compounded tacrolimus 0.1%), before undergoing four treatments of vascular beam laser therapy at 595 nm and long pulsed 532 settings. This induced purpura, but again failed to produce a significant effect. Following this, a small improvement was noted with seven treatments of intralesional corticosteroid administration consisting of 5-10 mg of triamcinolone acetonide 10 mg/ml Kenacort-A 10 (KA10, Aspen Pharmaceuticals, St Leonards, Australia). Central atrophy was noted, but little change was seen at the edge of the lesion which remained thick and prominent [Figure 4]. The response was considered inadequate by both the patient and clinician. Systemic therapies were briefly considered but avoided in this patient, due to his comorbidities and long-term use of carbamazepine for epilepsy control.
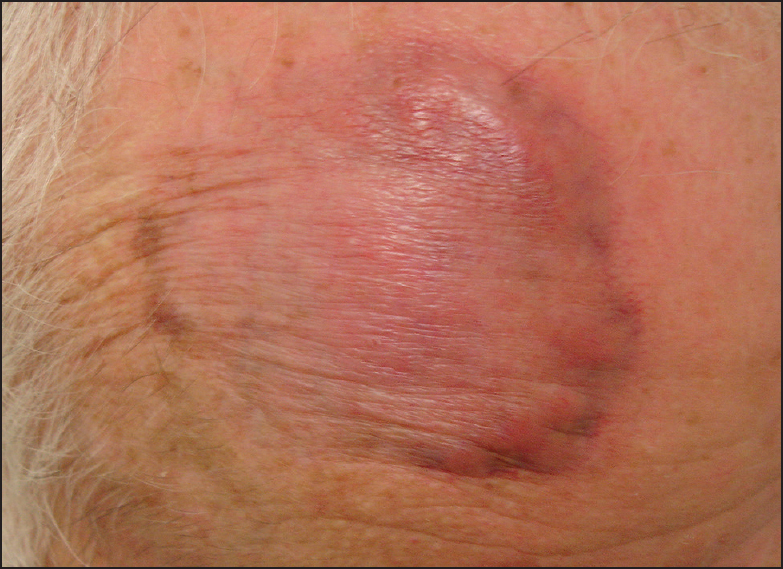
- Lesion following IL corticosteroid treatment only, prior to introduction of 5FU
The mild response of the lesion to intralesional steroids led to the idea of using intralesional 5-fluorouracil (5FU) as a steroid-sparing or replacement agent, in a similar way to the use of 5FU in hypertrophic and keloid scarring.[56] 5FU acts in a similar way to a steroid agent in keloid and hypertrophic scars, without the unwanted atrophic effects.[7] As first described by Fitzpatrick in the treatment of scars in 1999, an 80:20 mix of 80% 5FU (DBL Fluorouracil, 500 mg/10 ml, Hospira Australia Pty Ltd, Melbourne, Australia) and 20% KA10 was used to initiate treatment.[8] This yielded a mixture in a 1 ml syringe of 0.8 ml 5FU and 0.2 ml KA10. When initially reported, the small amount of steroid was added to the 5FU to limit the pain of injection.[8] The patient received intralesional KA10 and 5FU combination injections initially to the outer rim, and subsequently over the entire lesion. Various mixture quantities were used, ranging from 0.1 to 0.3 ml of KA10 given in combination with 0.7-0.95 ml of 5FU. These were given at intervals ranging from 2 weeks to 2 months according to patient availability and convenience.
The patient has now received a total of twenty injections over a period of more than 3 years. An excellent response has been noted and the patient is now able to tolerate long treatment-free periods between 9 and 12 months [Figure 5]. The small volume of KA10 relative to 5FU in the mixture suggests the response can primarily be attributed to the inclusion of 5FU, as the patient had not adequately responded to a pure steroid injection at higher concentration received initially.
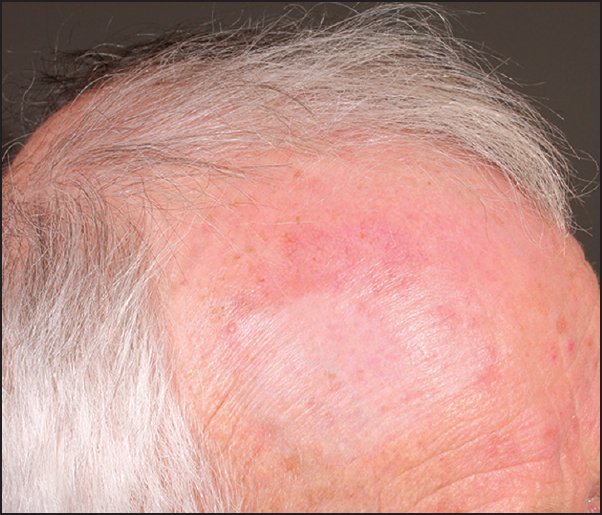
- Patient post treatment with combination KA10/5FU therapy
DISCUSSION
It is largely unknown why 5FU has been useful in this case. As a pyrimidine analogue, 5FU is transformed inside the cell to form cytotoxic metabolites that are incorporated into DNA and RNA, inducing cell cycle arrest and apoptosis. It is possible that 5FU had an apoptic effect on the inflammatory cells making up the infiltrate. 5FU is a simple and safe injection material and should be considered by clinicians as an option for treatment of GF. The response rate in patients with GF is not rapid (although similar to the rate seen with use in keloid scarring), and many injections are necessary to achieve success. The utility is in its’ ability to induce long periods of remission in the form of a disease-free interval, without the atrophy and rebound effects that are present with intralesional corticosteroids and other therapies.
Prior to commencing this treatment it is recommended that clinicians perform a blood screening tests including a full blood panel, renal and liver function tests. This should be repeated every 2-3 months following the first treatment. This treatment is unsuitable for pregnant or lactating women.
At the first session, a clinician should begin by using an 80:20 mix of 0.8 ml 5FU and 0.2 ml KA10. Total volume administered at subsequent sessions is at the clinician discretion and should be based on the size of the lesion and its response to the initial treatment. It is not recommended to use greater than 2 ml of an 80:20 mix per treatment.
This case demonstrates the success of intralesional 5FU in a subject with laser treatment resistant GF.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Pinkus’ Guide to Dermatohistopathology. (6th ed). East Norwalk, Conn: Appleton and Lange; 1995.
- [Google Scholar]
- Granuloma faciale: Successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-51.
- [Google Scholar]
- Comparison of the efficacy of intralesional triamcinolone acetonide and 5-fluorouracil tattooing for the treatment of keloids. Dermatol Surg. 2012;38:104-9.
- [Google Scholar]
- A study of the combination of triamcinolone and 5-fluorouracil in modulating keloid fibroblasts in vitro. J Plast Reconstr Aesthet Surg. 2013;66:e251-9.
- [Google Scholar]
- Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology. 2002;204:130-2.
- [Google Scholar]
- Treatment of inflamed hypertrophic scars using intralesional 5-FU. Dermatol Surg. 1999;25:224-32.
- [Google Scholar]






