Translate this page into:
Vitamin D Receptor Expression in Chronic Plaque Psoriasis Before and After Narrowband Ultraviolet B Phototherapy
Address for correspondence: Dr. Mayada Ismail, Department of Dermatology and Venereology, Faculty of Medicine, Tanta University, El Geish Street, Tanta 31111, Egypt. E-mail: mayadaia@hotmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background/Purpose:
Psoriasis is a multifactorial disease. It is a combination of genetic, immunological, and environmental factors. Vitamin D receptor (VDR) is a nuclear receptor that regulates epidermal cell growth through the inhibition of proliferation and induction of keratinocytes terminal differentiation. Aim of the study was to investigate the effect of Narrow-band UVB (NB-UVB) therapy on VDR expression in the skin of psoriasis patients.
Materials and Methods:
Forty patients with different severities of psoriasis were assessed using the psoriasis area and severity index (PASI) score. Lesional and non-lesional skin biopsies were obtained from each patient before NB-UVB therapy, and then a third lesional biopsy was performed after completing 24 sessions of NB-UVB. Immunohistochemistry for VDR was performed on all specimens.
Results:
There was a significant decrease in VDR expression in psoriatic lesions compared to that in non-lesional skin before treatment. A statistically negative correlation was detected between the degree of VDR expression before treatment and PASI score, family history, and duration of psoriasis. There was a significant increase in VDR expression at the sites of psoriasis lesions post-NB-UVB therapy compared to pretreatment lesional skin.
Conclusion:
VDR expression was down-regulated in psoriatic lesions compared to non-lesional skin, and NB-UVB therapy improved VDR expression in psoriasis skin lesions.
Keywords
NB-UVB
psoriasis
vitamin D receptor
INTRODUCTION
Psoriasis is a genetically determined immune-mediated, inflammatory skin disease.[1] Both T helper type 1 “Th1” and T helper type 17 “Th17” cytokines play a role in its pathogenesis. This cytokines mixture act on dermal and epidermal cells, altering the gene expression and maturation of keratinocytes and other cells.[2] Vitamin D is produced by keratinocytes following exposure to sunlight; it regulates multiple immunological functions, in addition to skeletal ones.[3] Vitamin D receptor (VDR) is a nuclear receptor that mediates most of the known functions of 1,25-dihydroxyvitamin D (1,25(OH)2D3), the active metabolite of vitamin D[4], that acts mainly on the VDR to regulate the epidermal keratinocyte growth and differentiation.[5] It also inhibits the production or the expression of the Th1 and Th17 cytokines.[6] Resting T-cells express mostly undetectable VDR levels, which increase as T-cells proliferate in response to antigenic activation.[7] In absence of the VDR, Th17 and Th1 cells are highly pathogenic.[8]
Vitamin D deficiency is associated with psoriasis and could play a significant role in its pathogenesis.[9] The clinical significance of hypovitaminosis D in psoriasis as well as the role and mode of its administration are topics currently under study.[3] Phototherapy increases the levels of serum 25(OH)D in patients with psoriasis,[10] and it has been proposed that narrowband ultraviolet (UV) B radiation may mediate its beneficial effect on psoriasis also by increasing endogenous vitamin D levels.[111213]
The observation that keratinocytes and T cells express VDR and that 1,25(OH)2D is a potent stimulator of keratinocyte differentiation provides a potential basis for the clinical use of VDR ligands for the treatment of psoriasis.[1415]
SUBJECTS AND METHODS
Patients
The current study included 40 patients with psoriasis; collected from the Outpatient Clinic of Dermatology and Venereology Department, Ethical Committee approval number.
Inclusion criteria
Patients were diagnosed clinically with chronic plaque psoriasis and confirmed histopathologically by hematoxylin and eosin (H&E) stained section.
Exclusion criteria
-
(1)
Patients who had any other dermatological or systemic diseases, pregnant, and lactating females were excluded.
-
(2)
Patients who received phototherapy, systemic, or topical therapy in the three months before the study were also excluded.
Clinical assessment
All patients were evaluated by psoriasis area and severity index (PASI) score as follows: <10 (mild psoriasis), 10–20 (moderate psoriasis), and >20 (severe psoriasis).
METHODS
Study protocol
As the aim of this study was to evaluate the effect of NB-UVB on VDR expression in psoriasis before and after NB-UVB treatment, after signing informed consent, each of the included patients underwent three (3 mm) punch biopsies under lidocaine 2% local anesthesia. Two biopsies were obtained before treatment, one from lesional skin to evaluate VDR expression before treatment, and the other biopsy from non-lesional skin to serve as the control. Then from lesional skin only, a third skin biopsy was retrieved after 24 sessions of NB-UVB with clinical improvement. The specimens were stained with H&E to confirm the clinical diagnosis of psoriasis.
Immunohistochemistry
Staining was done to detect VDR expression in non-lesional and lesional skin before and after NB-UVB by a streptavidin–biotin amplified system. The primary antibody was rabbit polyclonal to VDR (Novus Biologicals, code number NBP1-6794 USA; 1:100 dilution). In this system, two reagents were utilized: (1) the biotinylated secondary anti-immunoglobulin, which was a purified goat polyvalent anti-rabbit IgG capable of binding to the primary antibody and (2) and the streptavidin–biotin enzyme complex. The reaction was visualized by an appropriate substrate/chromogen (diaminobenzidine) reagent.
Evaluation of and interpretation of vitamin D receptor immunostaining
The percentage of stained cells was calculated by counting the number of stained epidermal cells in relation to the total number of the cells according to Visconti et al.[16]
-
-
Mild positivity (+1): <25% of keratinocytes showed positive staining.
-
-
Moderate degree of positivity (+2): 25–50% of keratinocytes showed positive staining.
-
-
Marked degree of positivity (+3): >50% of keratinocytes showed positive staining.
Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS Statistical Package of School Science (SPSS) Inc., Chicago, IL, USA, software package version 20.0. Qualitative data were described using numbers and percentages. Quantitative data were described using range (minimum and maximum), mean, standard deviation, and median. The significance of the obtained results was judged at the 5% level. Student t-test was used during comparison between the means of different sample groups., Chi-Square test was used test the association between qualitative nominal variables. Fisher’s exact test, Monte Carlo test, were used for calculation of the P value directly, without the use of particular test statistic, for correction for chi-square when more than 20% of the cells have expected count less than 5. Mann Whitney test for abnormally quantitative variables, to compare between two studied groups. Chi square for Kruskal Wallis test for abnormally quantitative variables, to compare between more than two studied groups. Spearman correlation coefficient to correlate between two abnormally quantitative variables. Significance was detected if p < 0.05.
RESULTS
Clinical results
The current work comprised of forty patients, 30 males (75%) and 10 females (25%). The clinical characteristics of the studied patients were demonstrated in Table 1.
| Parameters of clinical data | Patients (n = 40) | |
|---|---|---|
| No | % | |
| Sex | ||
| Male | 30 | 75.0 |
| Female | 10 | 25.0 |
| Age | ||
| ≤40 | 14 | 35.0 |
| >40 | 26 | 65.0 |
| Min.–Max. | 16.0–66.0 | |
| Mean ± SD | 46.75 ± 15.03 | |
| Median | 51.0 | |
| Duration | ||
| Min.–Max. | 1.0–30.0 | |
| Mean ± SD | 9.03 ± 10.62 | |
| Median | 4.0 | |
| Age of onset | ||
| ≤40 | 20 | 50.0 |
| >40 | 20 | 50.0 |
| Min.–Max. | 6.0–64.0 | |
| Mean ± SD | 39.93 ± 16.81 | |
| Median | 40.25 | |
| Family history | ||
| Negative | 26 | 65.0 |
| Positive | 14 | 35.0 |
| PASI score | Patients (n = 40) | |
| No | % | |
| Mild | 14 | 35.0 |
| Moderate | 16 | 40.0 |
| Severe | 10 | 25.0 |
| Min.–Max. | 1.40–49.0 | |
| Mean ± SD | 15.72 ± 11.93 | |
| Median | 12.75 | |
Immunohistochemical results of VDR expression
VDR expression was evaluated according to Viscont et al.[16] in the studied patients as follows:
For non-lesional skin
VDR was expressed in the nuclei of the epidermal cell layers of all specimens. 18 (45%) with a moderate degree of positivity (+2), and 22 (55%) with a marked degree of positivity (+3) [Figure 1A and Table 2].
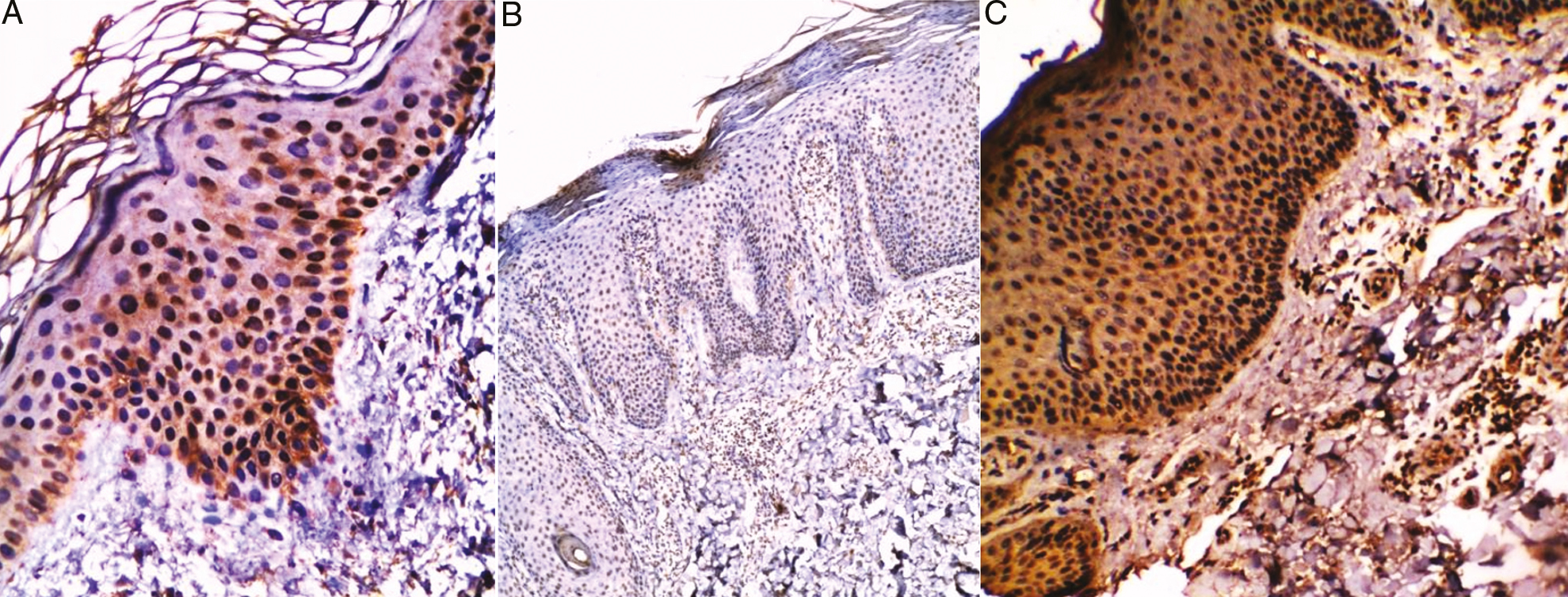
- (A) Non lesional skin of chronic plaque psoriasis showed marked (+3) expression of VDR in both basal cell layer and prickle cell layer (streptavidin–biotin ×200). Chronic plaque psoriasis (lesional skin) (B) before treatment with NB-UVB showed mild (+1) expression of VDR in basal cell layer (streptavidin–biotin ×40), (C) after treatment with NB-UVB showed marked (+3) expression of VDR all layers of epidermis (streptavidin–biotin ×100)
| VDR | ||||||
|---|---|---|---|---|---|---|
| Non lesional skin (n = 40) | Lesional skin before NB-UVB (n =40) | Lesional skin after NB-UVB (n = 40) | ||||
| No. | % | No. | % | No. | % | |
| Negative | 0 | 0.0 | 6 | 15.0 | 0 | 0.0 |
| Mild | 0 | 0.0 | 8 | 20.0 | 6 | 15.0 |
| Moderate | 18 | 45.0 | 16 | 40.0 | 16 | 40.0 |
| Marked | 22 | 55.0 | 10 | 25.0 | 18 | 45.0 |
| Non lesional versus lesional skin before NB-UVB | MHχ2 | 3.411* | ||||
| P | 0.001* | |||||
| Lesional skin before versus after NB-UVB | t | 4.819 | ||||
| P | 0.000* | |||||
MHχ2: Chi square for marginal homogeneity test
* Statistically significant at P ≤ 0.05
For lesional skin of psoriatic patients before NB-UVB
VDR was expressed in the nuclei of epidermal cell layers in 34 patients (85%) with different degrees of positivity. Eight of them (20%) with a mild degree of positivity (+1) [Figure 1B], 16 (40%) had a moderate degree of positivity (+2), and ten (25%) with a marked degree of positivity (+3) [Table 2].
VDR expression was statistically decreased in lesional skin before NB-UVB compared to non-lesional skin of the studied psoriatic patients (P value = 0.001*) [Table 2].
For lesional skin of psoriatic patients after NB-UVB
VDR was expressed in the nuclei of epidermal cell layers in all the patients (100%) with different degrees of positivity. Six of them (15%) with a mild degree of positivity (+1), 16 (40%) had a moderate degree of positivity (+2), and 18 (45%) with a marked degree of positivity (+3) [Figure 1C and Table 2].
Relation between VDR expression in lesional skin before treatment and clinical parameters of the patients
The VDR expression did not show any statistically significant difference regarding gender or age of the patients or age of onset of the disease.
The strength of VDR expression was negatively correlated with the following: duration of the disease (P value = 0.036*), the family history of the disease (P value = 0.032*), and PASI score (P value = 0.001*).
Relation between VDR expression in lesional skin after treatment with NB-UVB and before treatment.
VDR expression was statistically increased in lesional skin post-treatment with NB-UVB compared to lesional skin before treatment (P value = 0.000*) [Table 2] [Graph 1].
- Correlation between VDR expression in lesional skin before and after treatment with NB-UVB
DISCUSSION
The current study was conducted on forty patients with different severities of chronic plaque psoriasis, immunohistochemistry staining of VDR was performed for perilesional and lesional skin before NB-UVB therapy, then for lesional skin after completing sessions of NB-UVB therapy.
VDR expression was significantly increased in lesional skin after NB-UVB sessions compared to pretreatment level, P = 0.000*. Up to the best of our knowledge, there is no available literature about the immunohistochemical expression of VDR in psoriasis patients after exposure to NB-UVB compared to pretreatment level. These results may suggest an additional mechanism for the therapeutic effect of NB-UVB on psoriasis. El-Hanbuli et al.[17] detected improved VDR expression in the skin and serum 25-hydroxyvitamin D [25(OH)D] in vitiligo patients after NB-UVB therapy.
Studies done by Ryan et al.,[11] Wheinhold et al.[18] and Gupta et al.[19] found that NB-UVB (310–315 nm) treatment of psoriasis increased serum 25(OH)D levels together with PASI score improvement.
Chen et al.[20] detected increased VDR mRNA in psoriasis lesions treated with 1,25(OH)2D3 compared to lesions treated with Vaseline, While Ala-houhala et al.[21] demonstrated that serum 25-OH vitamin D levels increased by 58% from baseline when patients received oral vitamin D concurrently.
Vitamin D via VDR exerts an immunosuppressive, anti-proliferative, and pro-differentiation effect on the skin. Thus, VDR may play a role in controlling immune-mediated diseases, including psoriasis through its effect on regulating the functions of the epidermal barrier and/or local immune response.[22] It is known that vitamin D3 inhibits the production of IL-2 and IL-6, blocks transcription of IFN-γ and granulocyte-macrophage colony-stimulating factor mRNA, and inhibits cytotoxic T cells and natural killer cell activity.[4]
In the present study, VDR expression was detected in the nuclei of epidermal cell layers in psoriasis lesions and non-lesional skin except in the stratum corneum. These results agreed with the results of Milde et al.[23] and Solvsten et al.[24]
The present study detected a statistically significant decrease of VDR expression in lesional skin compared to non-lesional skin of psoriatic patients (P value = 0.001*). These results agreed with the studies done by Kim et al.[25] and Khalil et al.[26]
Visconti et al.[16] found a reduction of VDR expression in psoriasis skin compared to normal skin. This could be explained by the anti-proliferative effect of the VDR on keratinocytes. Also, they suggested a new role of VDR in the maintenance of the homeostasis of the skin barrier through its ability to modulate junctional protein expression.[16] This could be supported by the in vitro culture systems done by Kong et al.[27] treated with 1,25 (OH)2D3 for 24h, which showed increased levels of tight junction proteins ZO-1 and claudin-1.
Contrary to our results, Milde et al.[23] showed a significant increase in VDR expression in basal and suprabasal epidermal keratinocytes and the skin immune system cells in lesional psoriatic skin compared to non-lesional skin. The keratinocytes, activated macrophages, and melanoma cells have the ability to convert 25-hydroxyvitamin D3 into the active metabolite 1,25(OH)2D3in vitro. This might explain the role of 1,25(OH)2D3 in normal differentiation and immune response in the skin.[23]
The results of the current study disagreed with the results of Jensen et al.[28] and Solvsten et al.[24] They did not detect any significant difference in VDR levels between uninvolved and involved psoriatic skin.
The discrepancy of the results of the studies done on VDR (onset or therapy of disease) could be explained on the basis of genetic variation of the studied groups, different sampling strategies, more specifically, different constituent ratios of patients in terms of clinical types of psoriasis, age of onset of the disease, family history, size of the population samples, and therapeutic response to different treatment modalities with different concentrations.[29]
The present study detected a significant negative correlation between VDR expression and PASI score, this could support the possible role of this receptor in the severity of psoriasis. This agreed with the results of Chandra et al.[30] While Morimoto et al.[31] found a significant negative correlation between serum concentration of 1,25-(OH)2D and the severity of psoriatic skin lesions. El-Farargy et al.[32] showed a significant negative correlation between serum 25-hydroxyvitamin D and PASI score, duration of disease. However, Nayak et al.[33] did not find a correlation between serum 25(OH) and PASI score
It has been found that, topical calcipotriol treatment increased the expression of VDR on keratinocytes, therefore its effect on the proliferation and differentiation of keratinocytes was more than on dermal inflammation.[2021,34]
On the other hand, Zuel-Fakkar et al.[35] and Okita et al.[36] did not reveal any correlation between VDR gene polymorphisms and psoriasis development.
There were statistically significant correlations between degree of VDR expression and both of family history of the disease (P value =0.032*) and duration of the disease (P value =0.036*). Dayangac-Erden et al.[37] Kaya et al.[38] Park et al.[39] concluded that VDR gene was associated with familial psoriasis
It was postulated that VDR gene polymorphism influences serum level of vitamin D and consequently has an impact on the genetic susceptibility to psoriasis[2240] Liu et al.[41] stated that there is a relation between VDR gene polymorphisms and susceptibility to psoriasis and response to topical vitamin D (calcipotriol). On the other hand, Li et al.[42] failed to find a relation between VDR gene polymorphisms and susceptibility to psoriasis
The current study did not detect any significant correlation between VDR expression and gender, age of patients or age of onset of psoriasis. This agrees with Morimoto et al.,[31] Okita et al.[36] Zuel-Fakkar et al.[35] and Weinhold et al.[18] but, Park et al.[39] reported a significant association between VDR genotype and mean age of psoriasis onset.
Based on the current study, it could be concluded that VDR may play a key role in the pathogenesis as well as the severity of psoriasis. Our results showed increased expression of VDR in psoriasis skin lesions after NB-UVB compared to pretreatment levels, which was correlated with clinical improvement of the lesions.
The limitation of the present work was the inability to evaluate VDR in non-lesional skin after treatment because a fourth biopsy couldn’t be taken from the studied patients due to ethical considerations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-10.
- [Google Scholar]
- Vitamin D and the immune system: New perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39:365-79.
- [Google Scholar]
- Vitamin D regulation of immune function in the gut: Why do T cells have vitamin D receptors? Mol Aspects Med. 2012;33:77-82.
- [Google Scholar]
- Direct detection of free vitamin D as a tool to assess risk conditions associated with chronic plaque psoriasis. J Prev Med Hyg. 2020;61:E489-95.
- [Google Scholar]
- Phototherapy with UVB narrowband, UVA/UVBnb, and UVA1 differentially impacts serum 25-hydroxyvitamin-D3. J Am Acad Dermatol. 2013;69:530-6.
- [Google Scholar]
- The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch Dermatol. 2010;146:836-42.
- [Google Scholar]
- Effect of narrowband ultraviolet B therapy on serum vitamin D and cathelicidin (LL-37) in patients with chronic plaque psoriasis. J Cutan Med Surg. 2014;18:43-8.
- [Google Scholar]
- Narrowband UVB treatment increases serum 25-hydroxyvitamin D levels in patients with chronic plaque psoriasis. Cutis. 2017;99:431-5.
- [Google Scholar]
- Demonstration of 1,25dihydroxyvitamin D3 receptors in human skin biopsies. J Clin Endocrinol Metab. 1980;51:1463-1465.
- [Google Scholar]
- Immunohistochemical expression of VDR is associated with reduced integrity of tight junction complex in psoriatic skin. J Eur Acad Dermatol Venereol. 2015;29:2038-42.
- [Google Scholar]
- Narrow-band UVB effects on cutaneous vitamin D receptor expression and serum 25-hydroxyvitamin D in generalized vitiligo. Photodermatol Photoimmunol Photomed. 2018;34:175-83.
- [Google Scholar]
- Prospective investigation of 25(OH)D3 serum concentration following UVB narrow band phototherapy in patients with psoriasis and atopic dermatitis. Anticancer Res. 2016;36:1439-44.
- [Google Scholar]
- Efficacy of narrow band ultraviolet B phototherapy and levels of serum vitamin D3 in psoriasis: A prospective study. Indian Dermatol J. 2016;7:87-92.
- [Google Scholar]
- Induction of vitamin D receptor mRNA expression in psoriatic plaques correlates with clinical response to 1,25-dihydroxyvitamin D3. J Invest Dermatol. 1996;106:637-41.
- [Google Scholar]
- Narrow-band ultraviolet B treatment boosts serum 25-hydroxyvitamin D in patients with psoriasis on oral vitamin D supplementation. Acta Derm Venereol. 2014;94:146-51.
- [Google Scholar]
- Meta-analysis of vitamin D receptor polymorphisms and psoriasis risk. Int J Dermatol. 2013;52:705-10.
- [Google Scholar]
- Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J Invest Dermatol. 1991;97:230-9.
- [Google Scholar]
- Normal levels of the vitamin D receptor and its message in psoriatic skin. J Investig Dermatol Symp Proc. 1996;1:28-32.
- [Google Scholar]
- Toll-like receptors and antimicrobial peptides expressions of psoriasis: Correlation with serum vitamin D level. J Kor Med Sci. 2010;25:1506-12.
- [Google Scholar]
- Vitamin D3 level and its receptor of patients with psoriasis: A case–control study. EuroMediterranean Biomed J. 2018;13:150-4.
- [Google Scholar]
- Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:208-16.
- [Google Scholar]
- The vitamin D3 receptor and retinoid X receptors in psoriatic skin: The receptor levels correlate with the receptor binding to DNA. Br J Dermatol. 1998;138:225-8.
- [Google Scholar]
- Vitamin D endocrine system and psoriasis vulgaris—Review of the literature. Acta Dermatovenereol Croat. 2009;17:187-92.
- [Google Scholar]
- Pilot study: Immunohistochemistry expression of vitamin D receptor associated with severity of disease in psoriasis patients. Int J Dermatol. 2020;59:1092-7.
- [Google Scholar]
- Inverse relation between severity of psoriasis and serum 1,25-dihydroxy-vitamin D level. J Dermatol Sci. 1990;1:277-82.
- [Google Scholar]
- Correlation between vitamin D serum level and severity of psoriasis. Menoufia Med J. 2020;33:1016-20.
- [Google Scholar]
- Atopic dermatitis and vitamin D: Facts and controversies. An Bras Dermatol. 2013;88:945-53.
- [Google Scholar]
- A study of ApaI and TaqI genotypes of the vitamin D receptor in Egyptian patients with psoriasis. Clin Exp Dermatol. 2011;36:355-9.
- [Google Scholar]
- Polymorphism of the vitamin D (3) receptor in patients with psoriasis. Arch Dermatol Res. 2002;294:159-62.
- [Google Scholar]
- Polymorphisms of vitamin D receptor gene in Turkish familial psoriasis patients. Arch Dermatol Res. 2007;299:487-91.
- [Google Scholar]
- Association between vitamin D receptor gene polymorphism and psoriasis among the Turkish population. Arch Dermatol Res. 2002;294:286-9.
- [Google Scholar]
- Vitamin D receptor polymorphism is associated with psoriasis. J Invest Dermatol. 1999;112:113-6.
- [Google Scholar]
- A-1012G promoter polymorphism of vitamin D receptor gene is associated with psoriasis risk and lower allele-specific expression. DNA Cell Biol. 2014;33:102-9.
- [Google Scholar]
- Vitamin D receptor gene polymorphisms are associated with psoriasis susceptibility and the clinical response to calcipotriol in psoriatic patients. Exp Dermatol. 2020;29:1186-90.
- [Google Scholar]
- Pooling analysis regarding the impact of human vitamin D receptor variants on the odds of psoriasis. BMC Med Genet. 2019;20:161-75.
- [Google Scholar]






