Translate this page into:
Importance of Choke Vessels in Injectable Fillers
Address for correspondence: Dr. Venkataram Mysore, The Venkat Center for Skin and Plastic Surgery, 3411, 1st Floor, Service Road, Next to BTS Bus Depot, Vijaynagar, Bengaluru 560040, Karnataka, India. E-mail: mnvenkataram@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Knowledge of facial anatomy is indispensable for dermatologists and plastic surgeons practicing aesthetic medicine, especially for those using fillers, as injection of fillers may be associated with serious complications such as vascular occlusion and blindness. Angiosome and choke vessels play an important role in vascular incidents occurring after filler injections. The objective of this article was to outline the anatomy and pathophysiology of choke vessels, a concept which is not well known to dermatologists.
Keywords
Angiosomes
choke vessels
delay phenomenon
facial danger zones
fillers
flap surgery
vascular occlusion
Choke vessels are dormant vessels, which open up in special vascular compromise situations, and help to alter the circulation.
• This altered circulation may help in ameliorating the severity of damage caused by vascular occlusion after fillers.
• Knowledge of the concept and details of choke vessels is therefore important for the dermatologist and plastic surgeon.

INTRODUCTION
Knowledge of facial anatomy is increasingly becoming important for the procedural dermatologist and plastic surgeon, particularly for those who use fillers. Injection of fillers is associated with complications such as vascular occlusion and blindness, and hence the knowledge of vascular anatomy is vital. This article highlights the important aspects of vascular anatomy—angiosomes and choke vessels. Although this concept is well known to the plastic surgeons, dermatologists are often unfamiliar with it.
An “angiosome” is a composite anatomical territory comprising the skin and underlying tissues vascularized by a specific source artery and its accompanying vein or veins. Its anatomical boundary is established by a perimeter of connecting vessels, which connect it with the adjacent angiosomes to form a continuous network. These connecting vessels, which connect adjacent angiosomes (both arterial and venous skin circulation), are called choke vessels.[1] These “choke vessels” ordinarily exist in a state of reduced caliber during routine circulation. In other words, they lie dormant. They have the ability to dilate and become active during times of greater demand to facilitate increased blood flow.[23] These vessels are possibly controlled by hormonal, nervous, or biochemical factors.[1]
Choke vessels are often confused with anastomoses. Both link adjacent vascular territories and perform similar function of allowing enhanced blood flow. However, anastomotic vessels have normal caliber, whereas choke vessels have reduced caliber during normal vascular supply. Figure 1 shows structure of both choke and true anastomoses.

- Choke vessels and true anastomoses connecting adjacent angiosomes. (A) Choke anastomoses between adjacent arteries (reduced vessel size). (B) True anastomoses between adjacent arteries (normal vessel size)
“Choke” vessels play a significant role during both physiological and surgical circumstances that occur during transfer of different types of flaps of skin, muscle, tendon, nerve, and bone in various plastic and reconstructive surgeries.[1] Behavior of choke vessels influences flap survival in these surgeries.
The relevance of choke vessels lies in the fact that they can play an important role in preventing or ameliorating complications following intra-arterial injection of hyaluronic acid fillers. This article contains discussion on the applicability of choke vessels in intra-arterial injection of dermal fillers.
Etiology of choke vessel dilatation
Choke vessels ordinarily experience minimal blood flow due to the opposing pressures from adjacent vascular networks.[4] When blood flow to a particular region of skin is interrupted, an adjacent cutaneous perfusion territory can expand via choke vessels to compensate for the insufficient blood flow. Hypoxia and physical effects of blood flow are the major factors responsible for choke vessel’s function and change in caliber.[56] Inflammation and mechanical force are other important contributing factors.[7]
Hypoxia-ischemia process: Hypoxia is the major driving force for the vascular changes in the choke zone.[5] At first, a sustained hypoxic-ischemic process takes place in the multi-territory perforator flap.[89] Initial 48–72h of ischemia lead to hyperplasia and hypertrophy of cells of the vessel walls.[510] This ischemia–reperfusion injury results in damage at the cellular and subcellular levels, which contributes to post-ischemic tissue necrosis.[11] The line of necrosis usually corresponds to the choke vessel zone between the adjacent angiosomes.[12]
Alteration in blood flow and mechanical forces: Choke vessel remodeling occurs through the process of arteriogenesis (sprouting of microvessels from a preexisting capillary network).[13] Arteriogenesis in collateral arteries is precipitated by mechanical forces.[141516] When a major conductance artery is blocked by embolism or injury, blood flow to angiosomes is disrupted and pressure differentials are created inside choke vessels. This causes increased shear stress on the choke vessel wall, followed by disruption of the tunica intima, the growth in number and size of vessel cells, elongation of the smooth muscle cells, arteriogenesis, and choke vessel growth.[5171819]
Inflammation: Inflammation plays a key role in the vascular changes occurring in the delay procedure (see the paragraph on “delay phenomenon”), and is indispensable for the blood vessels to grow by arteriogenesis.[1518,20] Numerous inflammatory mediators are involved in the choke vessel remodeling. Various studies have described the association of mechanical stress within the collateral arterioles with the production of various pro-inflammatory molecules, such as monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin-8, and interleukin-6.[2122] TNF-α, MCP-1, hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF), heat shock proteins (HSP), CD11b, ICAM-1 (intercellular adhesion molecule 1), and MMP-2 (matrix metalloproteinase 2) have all been shown to mediate choke vessel transformation.[723,24]
Morphological and histopathological features of choke vessel remodeling
Various experimental research have been conducted on rat skin flap models for morphological and histological analysis of choke zone in flap elevation surgery. Several tools such as histopathology, immunohistochemistry, X-ray angiography, and computed tomographic angiography have been used in these studies.[710,2526]
According to a study by Zhuang et al.,[27] both choke arteries and choke veins and communicating venules between the choke veins increase in diameter and tortuosity after flap elevation. Vessels expand in size first by passive dilation, followed by an active growth phase.[28] During the active growth phase, the walls of choke vessel enlarge by hypertrophy and hyperplasia. There is dilation of the choke vessel caliber with tortuous paths and increase in the vessel wall thickness 7 days after surgery with expansion of territory of artery to supply adjacent vascular territories.[23] Macroscopic changes such as corkscrew shape and territorial expansion are also seen at day 7 after flap elevation.[7] Figure 2 shows the sequence of changes in choke vessels pre- and post-flap elevation.
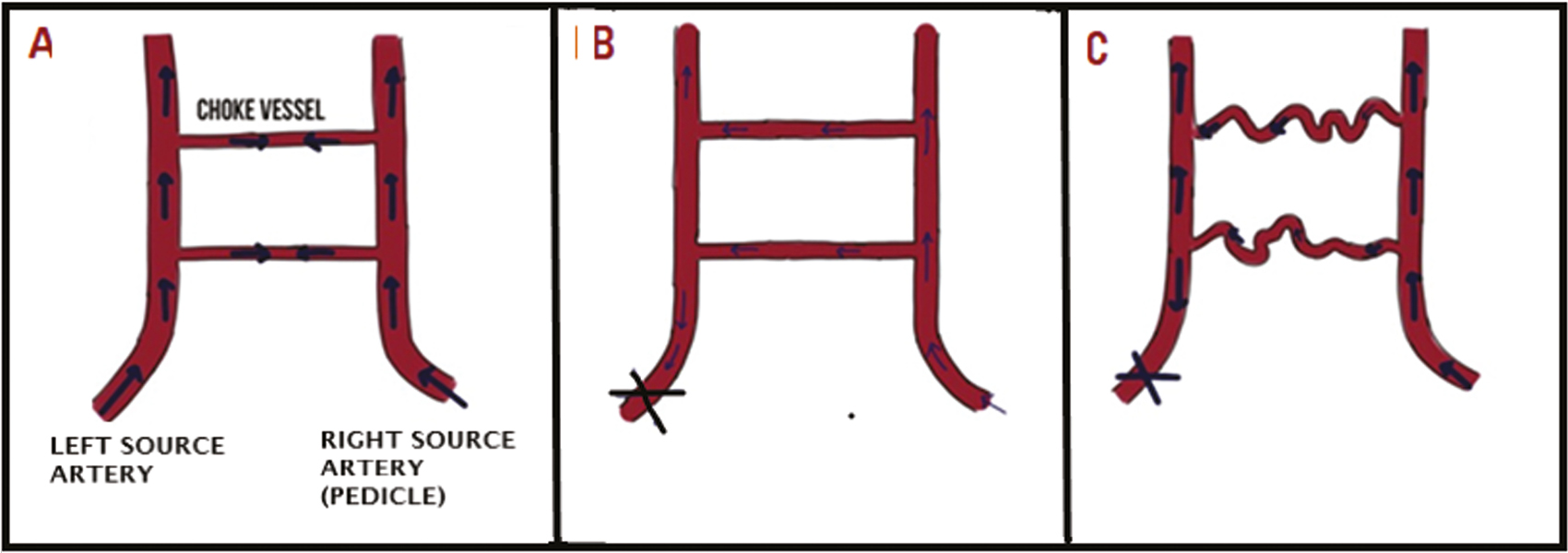
- Sequence of events with alteration in blood flow in a vascular territory flap before and 7 days after flap elevation. Figure shows the sequence of changes in choke vessels pre- and post-flap elevation in plastic and reconstructive surgeries. (A) Shows choke vessels under normal physiological conditions where they encounter balanced pressure from source arteries. (B) Immediately after flap elevation there is a disbalance in pressure in choke zone and there is increased blood flow in choke vessels. (C) 7 days after flap elevation choke vessels become tortous with increase in vessel wall thickness and diameter, transforming into functional arteries
Clinical relevance of choke vessel remodeling
Role of choke vessels in flap surgery and delay phenomenon
Choke vessels play a very important role in plastic, reconstructive, and cardiac surgery.[2729] Flap surgery is a technique used in plastic and reconstructive surgeries, which involves transportation of a healthy, live tissue from donor site to recipient site with an intact blood supply. Angiosomes are clinically relevant as they define the nominal volume of composite tissue that can be safely transferred on a source artery in these reconstructive flap surgeries.[1] The duration of survival of a flap depends on the number of choke zones linking adjacent territories.[2730,31]
The delay phenomenon, also known as vascular delay or ischemic preconditioning, is a theory stating that when flap tissue is made partially ischemic, it undergoes neovascularization and increased vascularity. In simple terms, it refers to “delaying” a flap transfer. Here a part of the flap is incised initially, thereby disrupting a fraction of the blood supply, without transferring the flap in this first stage. Soon after the flap elevation, choke vessels open up and dilate to the size of true anastomoses, thus compensating for the disrupted blood supply.[32] Thus, vascular delay helps the flap in two phases—early and late. In the early phase, there is a dilation and reorientation of choke vessels, due to the alteration in sympathetic tone, and modifications of metabolic pathways. Post-flap dissection, in the late phase, the choke vessels remodel along the long axis of the flap, with new vessel growth augmenting blood supply to regions of the flap most prone for necrosis [Figure 2].[1333,34] Once the choke vessels have opened up fully, it is safe to complete the flap incision and disrupt the rest of the blood supply and transfer the flap. All flaps experience some ischemia, and the technique of reconstructive surgery uses this concept of delay phenomenon to facilitate the survival of flaps.[353637]
Role of choke vessels in inadvertent intra-arterial injection of hyaluronic acid fillers
Though inadvertent intra-arterial hyaluronic acid injections are rare, they are well-documented.[383940] Intra-arterial injections are more prevalent when the needle is injected parallel to known branches of the facial artery, especially the labial artery in the lip, and the dorsal nasal vessels in the nose, or when a bolus of hyaluronic acid is injected with the tip of the needle remaining in a stationary position.[12]
Skin does not contain end arteries, and blood flow is controlled by the alteration of blood flow through choke vessels. There is either reduction of blood flow through small-caliber choke vessel or blockage of blood flow by spasm of choke vessels. Therefore, choke vessels can prevent entry of a filler material from an adjacent involved territory, and thereby maintain viability of the territory.[12]
Certain dermal fillers have potential to stimulate active intravascular inflammatory reaction, leading to increased magnitude of vascular obstruction in contrast to noninflammatory hyaluronic acid fillers, which cause pure mechanical effect.[41] When inflammation occurs in choke vessels, they respond with spasm, unlike other blood vessels, thereby restricting blood flow and diffusion of the hyaluronic acid filler into adjacent vascular angiosomes. Arteriovenous shunts open up to divert the hyaluronic acid into the venous system [Figure 3]. Thus, the location and role of these choke vessels is crucial in establishing the site of greatest impact and the extent of tissue necrosis.[12]
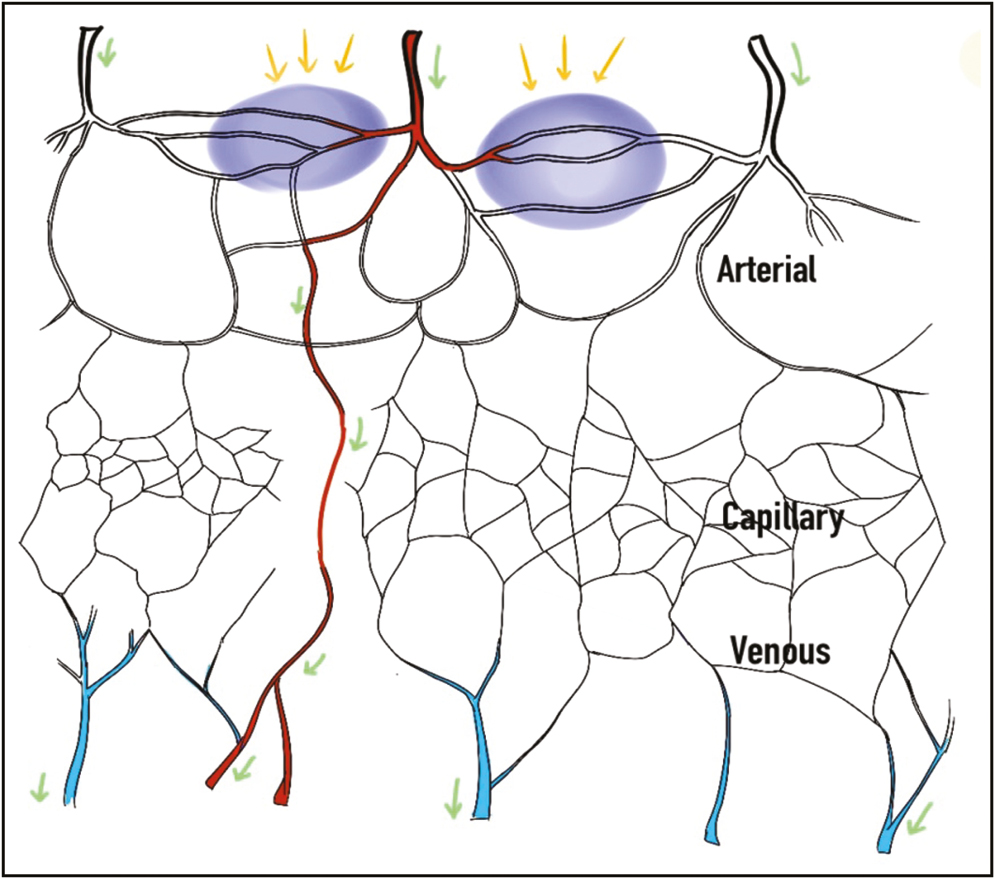
- Mechanism of prevention of hyaluronic acid embolus (blue) from extending into adjacent angiosomes by spasm of choke vessels (purple areas and yellow arrows) and its diversion into the venous system by arteriovenous shunting (green arrows)
Another area of choke vessel impact in filler patients is with respect to the use of nitroglycerin paste for vascular complications. Nitroglycerin is used due to its vasodilatory effect. However, its use is controversial as nitroglycerin paste can induce choke vessel dilation, which may then lead to release of the hyaluronic acid embolus into adjacent vascular territories, thereby expanding the number of angiosomes affected and consequently leading to an increase in the area of tissue necrosis.[12]
As choke vessels connect both arteries and veins, hyaluronic acid can accidently be pushed into the venous system or it may flow in retrograde fashion from arteries to veins and affect the wall of veins. According to a study by Taylor et al.,[42] due to paucity of valves in the facial veins, there is reverse flow of hyaluronic acid in veins, leading to its accumulation at sites such as lower border of the mandible and the glabellar region of the forehead.
The ability of facial veins to hold major portion of intravascular blood volume probably aids in the dilution of hyaluronic acid and leads to its flushing into larger vessels of the neck. Alternatively, due to inflamed facial veins, a filler injection into the venous system may flow in a retrograde fashion into the ophthalmic vein, cavernous sinus, and brain, where there are no protective valves.[43]Table 1 summarizes the components of inadvertent intra-arterial hyaluronic acid filler.
| 1. Embolus in the artery with inflammation of the vessel wall |
| 2. Spasm of the choke anastomoses around its anatomical perimeter to prevent tissue necrosis |
| 3. Spasm of true anastomoses, which may result in uninhibited passage of the filler at a remote site (even on the opposite side of the face), which may even cause blindness |
Choke vessels and volume of fillers
Volume of fillers to be injected at different facial sites is also pivotal with respect to complications. Large amounts of fillers into any one area can cause a proportionally greater degree of arterial obstruction, and therefore it is not safe to inject more than 0.1 mL into any one location.[41] When larger volume is needed at one location, it is better to do it in stages and change the position for further injections. Arterial/venous occlusion may be sequelae of intra-arterial injection of fillers or external compression by large amount of surrounding filler.
In the event of inadvertent intra-arterial injection of large bolus of filler material, the main artery will be blocked, and hence all the blood supply to that area of skin will be disrupted. In such a scenario, choke vessels may not be able to compensate, and there may be skin necrosis. Sometimes there may be retrograde flow of the filler material after it has filled in the distal segment, and it may be carried to distant sites.[41] Vision loss may occur due to occlusion of the ophthalmic artery (presumably via retrograde flow from the supratrochlear, supraorbital, and dorsal nasal artery) as aforementioned.[44]
However, if smaller volume of filler is injected, then there will be either partial blockage of the main vessel, or the bolus will travel downstream to a smaller vessel and cause partial vascular loss. In this case, choke vessels may be able to compensate for the lost blood flow, and the skin might be saved by the delay phenomenon as explained earlier.[41]Figure 4A and B depicts the effect of small filler bolus and large filler bolus on intravascular pathways, respectively.
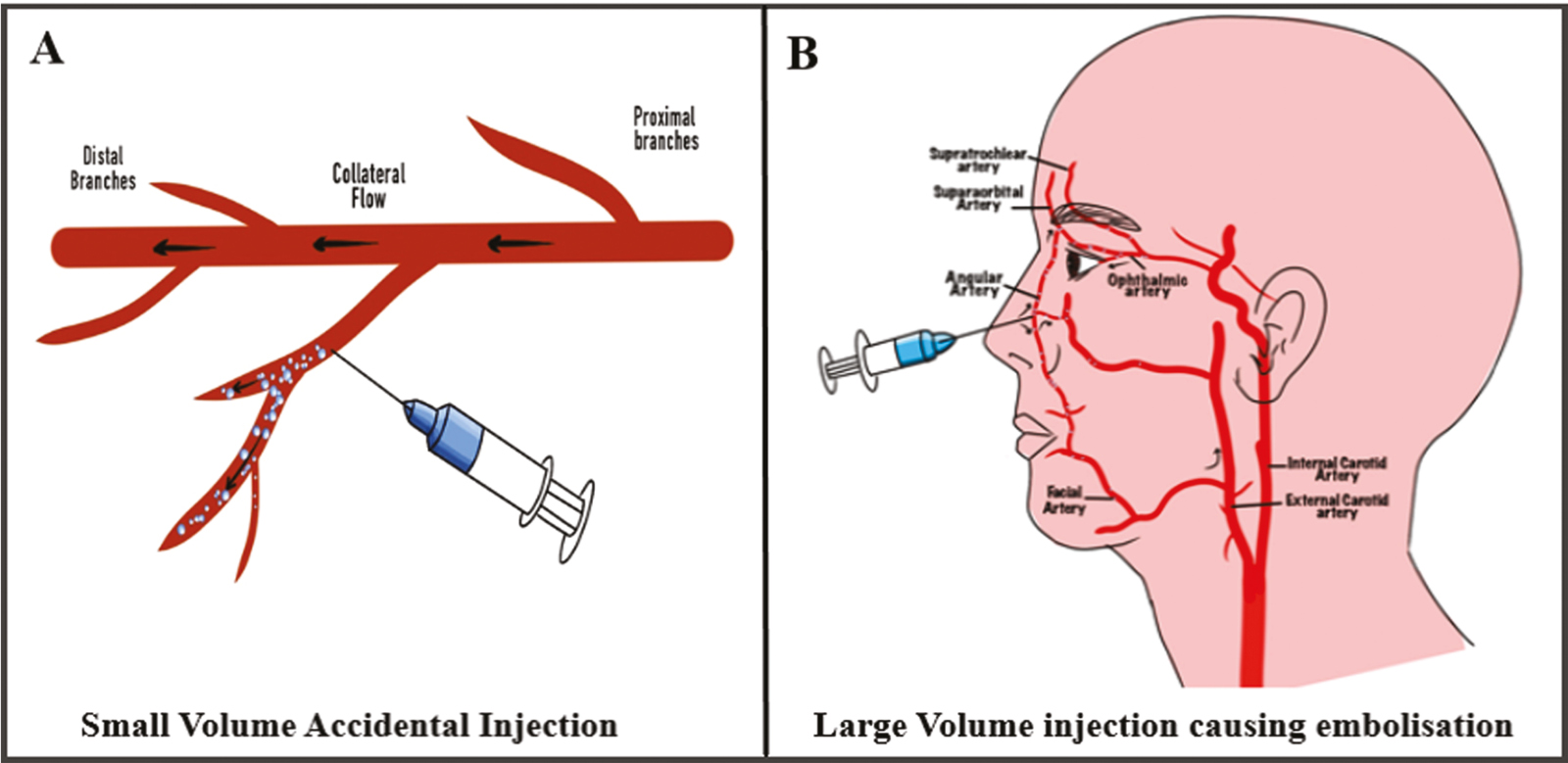
- (A) and (B) show the effect of small filler bolus and large filler bolus on intravascular pathways, respectively
Choke vessels and danger zones of the face
Maximum complications of fillers on face occur in the areas within the angiosomes of facial and ophthalmic artery arteries, often referred to as “danger zones of face,” irrespective of the site of injection. Figure 5 shows the facial “danger zones.” This territory of face constitutes the area of highest number of choke vessels.[1245] The concepts explained earlier are therefore particularly important in this region.
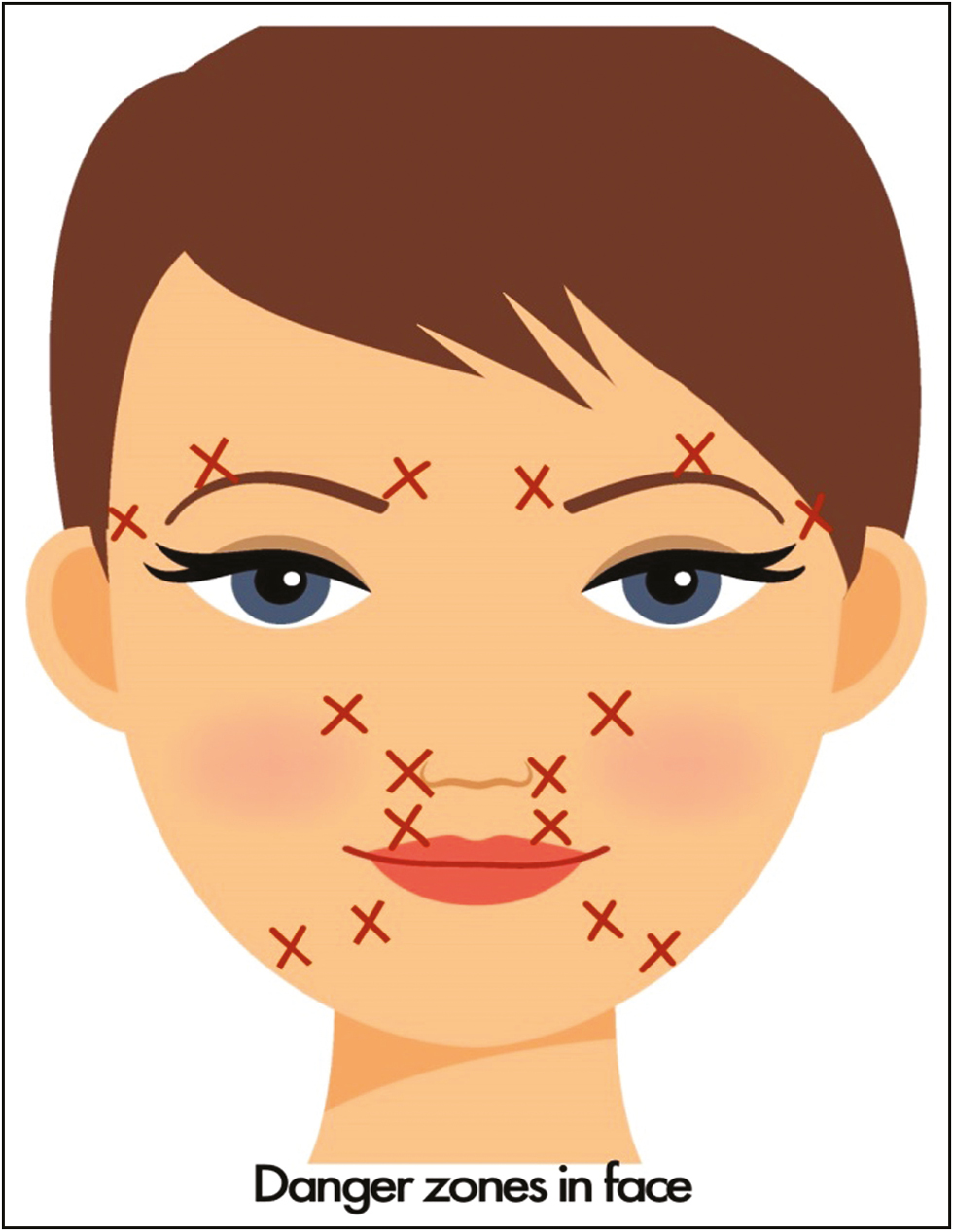
- Facial danger zones
CONCLUSION
For dermatologists and plastic surgeons using injectable fillers, knowledge of choke vessels is relevant and important. Choke vessels can play a role in limiting the damage of inadvertent vascular injection of filler. Proper knowledge of the anatomy of vessels, technique, and volume of injection, all have bearing on prevention and management of vascular complications occurring after fillers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We wish to thank Dr. Jeeno Jayan for contributing the figures used in the article that greatly improved the manuscript.
REFERENCES
- The vascular anatomy of rectus abdominis musculocutaneous flaps based on the deep superior epigastric system. Plast Reconstr Surg. 1988;82:815-32.
- [Google Scholar]
- The branching pattern of the deep inferior epigastric artery revisited in vivo: a new classification based on CT angiography. Clin Anat. 2010;23:87-92.
- [Google Scholar]
- The function of ‘choke vessels’ to the blood flow: angiographic and laser flow-graphic study on the rat flap model. Wound Repair Regen. 2004;12:A15.
- [Google Scholar]
- Impact of arteriogenesis in plastic surgery: choke vessel growth proceeds via arteriogenic mechanisms in the rat dorsal island skin flap. Microcirculation. 2009;16:235-50.
- [Google Scholar]
- Detrimental effect of hypoxia-inducible factor-1α-induced autophagy on multiterritory perforator flap survival in rats. Sci Rep. 2017;7:11791.
- [Google Scholar]
- Regulation of angiogenesis by oxygen sensing mechanisms. J Mol Med (Berl). 2009;87:549-60.
- [Google Scholar]
- The time sequence of the delay phenomenon: when is a surgical delay effective? An experimental study. Plast Reconstr Surg. 1995;95:526-33.
- [Google Scholar]
- Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229-317.
- [Google Scholar]
- The role of anastomotic vessels in controlling tissue viability and defining tissue necrosis with special reference to complications following injection of hyaluronic acid fillers. Plast Reconstr Surg. 2018;141:818e-30e.
- [Google Scholar]
- Cellular and molecular mechanisms of hypoxia-inducible factor driven vascular remodeling. Thromb Haemost. 2007;97:774-87.
- [Google Scholar]
- Arteriogenesis proceeds via ICAM-1/Mac-1- mediated mechanisms. Circ Res. 2004;94:1179-85.
- [Google Scholar]
- Collateral growth in the peripheral circulation: a review. Vasc Endovascular Surg. 2004;38:291-313.
- [Google Scholar]
- Arteriogenesis: mechanisms and modulation of collateral artery development. J Nucl Cardiol. 2001;8:687-93.
- [Google Scholar]
- Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949-71.
- [Google Scholar]
- Arteriogenic growth factors, chemokines and proteases as a prerequisite for arteriogenesis. Drug News Perspect. 2005;18:317-22.
- [Google Scholar]
- Induction of IL-8, MCP-1, and bFGF by TNF-alpha in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammation. Graefes Arch Clin Exp Ophthalmol. 2004;242:409-13.
- [Google Scholar]
- Inflammatory response associated with choke vessel remodeling in the extended perforator flap model. Exp Ther Med. 2017;13:2012-8.
- [Google Scholar]
- The hemodynamic and molecular mechanism study on the choke vessels in the multi-territory perforator flap transforming into true anastomosis. Gene. 2019;687:99-108.
- [Google Scholar]
- Three-dimensional anatomical vascular distribution in the pectoralis major myocutaneous flap. Plast Reconstr Surg. 2005;115:1342-52. discussion 1353-4
- [Google Scholar]
- Three- and four-dimensional computed tomographic angiography and venography of the anterolateral thigh perforator flap. Plast Reconstr Surg. 2008;121:1685-96.
- [Google Scholar]
- A novel in vivo technique for observations of choke vessels in a rat skin flap model. Plast Reconstr Surg. 2012;130:308-17.
- [Google Scholar]
- Role of ischemia and of hypoxia-inducible genes in arteriogenesis after femoral artery occlusion in the rabbit. Circ Res. 2001;89:779-86.
- [Google Scholar]
- “Choke” vessels between vascular territories of the abdominal wall: literature review and rare case of Leriche’s syndrome. Clin Anat. 2012;25:998-1004.
- [Google Scholar]
- An anatomic review of the delay phenomenon: I. experimental studies. Plast Reconstr Surg. 1992;89:397-407.
- [Google Scholar]
- An anatomic review of the delay phenomenon: II. clinical applications. Plast Reconstr Surg. 1992;89:408-16. discussion 417-8
- [Google Scholar]
- The functional angiosome: clinical implications of the anatomical concept. Plast Reconstr Surg. 2017;140:721-33.
- [Google Scholar]
- Tissue oxygen measurements in delayed skin flaps: a reconsideration of the mechanisms of the delay phenomenon. Plast Reconstr Surg. 1988;82:328-36.
- [Google Scholar]
- The delay phenomenon: a compilation of knowledge across specialties. Craniomaxillofac Trauma Reconstr. 2014;7:112-8.
- [Google Scholar]
- Differences in the delay phenomenon in the rabbit, rat, and pig. Plast Reconstr Surg. 1971;47:73-8.
- [Google Scholar]
- Augmentation of tissue survival by delay: an experimental study in rabbits. Plast Reconstr Surg. 1967;39:397-401.
- [Google Scholar]
- Injection necrosis of the glabella: protocol for prevention and treatment after use of dermal fillers. Dermatol Surg. 2006;32:276-81.
- [Google Scholar]
- Clinical experience with hyaluronic acid-filler complications. J Plast Reconstr Aesthet Surg. 2011;64:892-6.
- [Google Scholar]
- Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J. 2014;34:584-600.
- [Google Scholar]
- The venous territories (venosomes) of the human body: experimental study and clinical implications. Plast Reconstr Surg. 1990;86:185-213.
- [Google Scholar]
- An islanded rabbit auricular skin flap model of hyaluronic acid injection-induced embolism. Aesthetic Plast Surg. 2016;40:421-7.
- [Google Scholar]
- Avoiding the “danger zones” when injecting dermal fillers and volume enhancers. Plast Surg Nurs. 2014;34:108-11. quiz 112-3
- [Google Scholar]





