Translate this page into:
Reconstruction of Scrotal Skin Defects Using Bilobed Pudendal Flap
Address for correspondence: Dr. Malik Abaci, Training and Research Hospital of Karabük University, Demirçelik Kampüsü Tıp Fakültesi Dekanlığı Kat 4, 78050, Karabük, Turkey. E-mail: md.malikabaci@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Due to its unique cosmetic appearance and functioning, scrotal skin offers a major clinical challenge in terms of reconstruction. Thus, successful reconstruction of scrotal skin should include both provision of pliable texture and protection of testicular functions. Skin grafts and flaps are important options for such reconstruction; however, they both have unique features that bring about specific limitations and specific problems.
Aims and Objectives:
In the light of the negativities related to the widely used skin grafts and flaps, this study aims to discuss the use of bilobed pudendal flap—a simple, uniform, and pliable tissue—for the first time in the related literature for the reconstruction of scrotal skin defects caused by Fournier’s gangrene.
Materials and Methods:
This study was performed using the single-step method of scrotal skin reconstruction on eight patients who had developed scrotal skin defects and underwent reconstruction by using three-dimensional bilobed pudendal flaps (defect and reconstructive tissues planned on different planes) from December 2016 to August 2019.
Results:
No complication such as infection, bleeding, hematoma, partial or complete flap loss, scar contraction, urinary problem, erectile dysfunction or discomfort, or sensation loss was observed in seven out of eight study patients. The only complication to have developed in one patient was minimal dehiscence, which was then corrected by restoration.
Conclusion:
Repair of scrotal skin defect using a three-dimensional bilobed pudendal flap enabled an elastic scrotal repair acceptable in sensorial and visual terms.
Keywords
Bilobed flap
pudendal flap
scrotal defect
scrotum reconstruction
INTRODUCTION
Scrotal skin defects generally occur secondary to burn injuries, infection, and tumor ablation. Among these factors, Fournier’s gangrene is a type of necrotizing fasciitis harming particularly the soft tissues in the genital area. It has predisposing factors such as diabetes mellitus, alcohol use, obesity, local traumas, and perineal and perirectal infections. In these patients, both aerobic and anaerobic bacteria are the causative agents and the disease rapidly spreads to involve the surrounding soft tissue. Early debridement and antibiotherapy are the most important steps in the treatment of rapidly progressing fasciitis. Testes, having blood supplies independent of the soft tissue, can usually remain viable after these debridement procedures; however, they remain open after debridement and should be covered by an appropriate tissue.[123] A successful repair should retain testicular viability as well as achieving appropriate covering tissue. Parallel to this, a proper repair is of great importance for a patient’s safety and psychology as the penis and testes are the primary sexual organs of males. Therefore, care should be taken to restore the penis or scrotum to its original shape, and it would be advantageous to use a flap of appropriate thickness for reconstruction. There are several techniques for scrotal coverage. To this end, skin grafts and various flap options are widely recommended for the reconstruction of scrotal skin defects.[34567] In this parallel, previous studies have used testes embedded in the skin, skin grafts, local fasciocutaneous flaps, local perforators flaps, musculocutaneous flaps, and tissue expanders, with their specific advantages and disadvantages.[3456789101112131415161718] For example, friction may develop (depending on the features of the perineal area) during the reconstruction using skin grafts, possibly resulting in avulsion and graft infection. Black et al.[19] and Alwaal et al.[20] reported contracture development and pain in perineal reconstruction using a skin graft. Although offering good sectoral repair, skin grafts are also reported to frequently result in scar formation, leading to pain.[19] In addition, while playing an important role in the reconstruction of large scrotal skin defects, skin grafts are not advantageous in terms of thermoregulation.[13] As mentioned above, another option is the use of various types of flaps. But as is in skin grafts, flaps bring some problems as well. For example, musculocutaneous flaps dissected from the medial femoral have been frequently used before, however, proved to be too bulky.[2122] In addition, Young and Wright[21] and Kayikçioğlu[22] stated that musculocutaneous flaps such as gracilis, rectus abdominis, and tensor fascia flaps can be used but create discomfort in the narrow perineal area due to their thickness. Thus, they advised a second session for defatting and/or excision procedures aimed to reduce the bulky tissues of the flap.
In addition, the study by Atik et al.[4] showed that the use of local fasciocutaneous random flaps results in circulatory disorder due to proximity to the trauma zone, and these flaps are thick due to their lipid content. In the study by Wang et al.,[23] spermatogenesis was evaluated in the patients to have undergone scrotum construction using skin flap. The study showed that sperm count and activity decreased in flaps as the temperature cannot be retained in thick-flap reconstructions, as normal spermatogenesis requires the temperature to be kept in the range 2–8°C. Flaps such as anterolateral thigh flap and medial circumflex femoral artery perforator flap obtained from inguinal, gluteal, and medial femoral areas have been tried as well to achieve thinner flaps but found to introduce various problems including bulkiness.[523] Hallock et al.[3] and Coskunfirat et al.[5] performed scrotal reconstruction using medial circumflex femoral artery perforator flap; they reported the need for the further microsurgical technical procedure, flap-color incompatibility, and being not a directly sensory flap as the disadvantages of using the concerned flap. In the study on anterolateral thigh flap, Wang et al. observed traction of flap pedicle due to regional distance, which gives rise to the idea that leg abduction can manipulate the result of reconstruction. Perforator flaps and free flaps also have been commonly used in previous studies.[34511] However, free flap repairs involve a long surgical process. In addition, such procedures require an experienced microsurgery team and a close postoperative follow-up. Interstitial edema may occur in island-shaped perforator flaps and free flaps due to disruption of lymphatic and venous drainage. This situation results in the thickening of the flap and, thus, reduces its pliability and functionality. Moreover, interstitial lymphatic edema may develop in perforator flaps or single-lobe flaps, which can result in contractures by reducing pliability. Flap planned from the opposite scrotum would be a good option if it could cover wide defects.[24] In the study, Karaçal et al.[25] used a single-lobe pudendal flap to reconstruct scrotal skin defect. Single-lobe flaps have been reported to sufficiently repair small defects while remaining insufficient for large-scale defects.
Jeong et al.[24] repaired only minor defects using a directly sensory flap with satisfying color match from the opposite scrotum; however, they reported unusability of this method in the reconstruction of large-size defects.
The two-stage reconstruction using tissue expanders is another option for genital area reconstruction; however, it may fail due to severe Fournier’s gangrene infection and the difficulty of application in the genital area.[1017] Scrotum generally does not provide a sufficient amount of healthy skin for widening, which is another limitation of the tissue wideners.
In the light of the potential unsatisfactory results offered by the existing options for scrotal skin reconstruction, this retrospective study introduces the use of bilobed pudendal flap in the reconstruction of scrotal skin defects caused by Fournier gangrene, as it is a thin and durable flap free from the above-mentioned disadvantages. Although previously used in the urogenital region, the bilobed pudendal flap has never been used for scrotal defect repair before. So, this is the first study to analyze the effectiveness of the flap planned from the pudendal thigh skin, which appears to be the best match for scrotal reconstruction, in terms of thickness, color, and structure.
MATERIALS and METHODS
Study participants
Eight (n = 8) patients with Fournier’s gangrene-induced total scrotal skin defect reconstructed using bilobed pudendal flaps in 2016–2019 were retrospectively evaluated. The mean age of patients was 63 years (range, 50–68 years). All patients had comorbidities, and each of them had been previously diagnosed with diabetes mellitus; some also had hyperlipidemia and coronary artery disease. All study patients were followed up and treated with debridement and antibiotherapy for Fournier’s gangrene in the urology clinic of the Training and Research Hospital of Karabuk University.
Anatomical analysis of flap pedicle
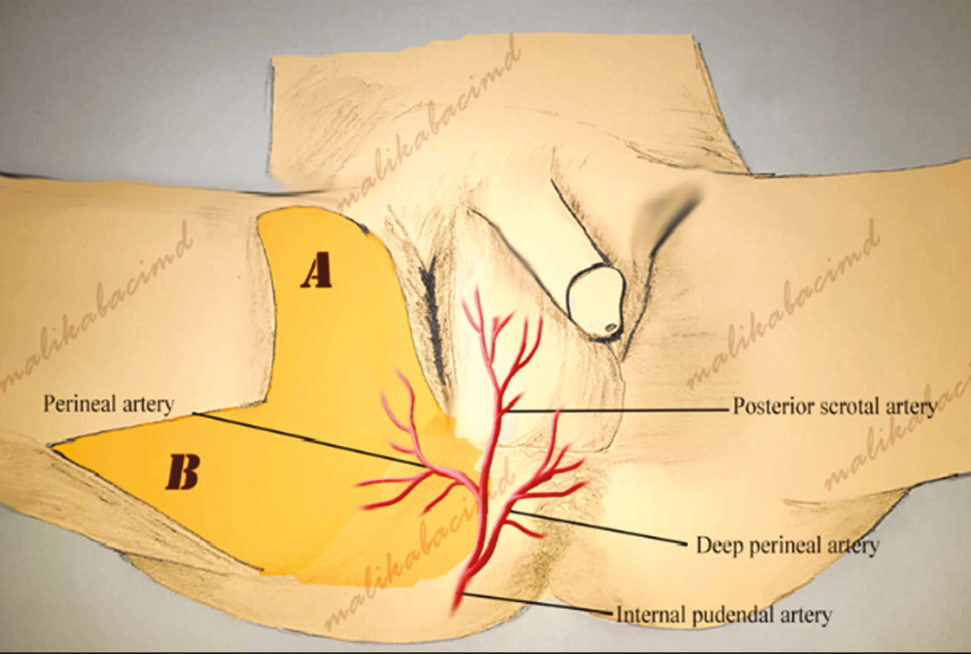
The anatomy of the internal pudendal artery has been defined in detail by a previous study.[26] The internal pudendal artery is one of the three branches of the anterior trunk of the internal iliac artery and is the primary supply of the external genitalia. It is smaller in females than in males. This artery travels through the pudendal canal with the internal pudendal veins and the pudendal nerve. The internal pudendal artery branches off the iliac artery within the pelvis, crosses the sacrotuberous ligament, and traverses the ischiorectal fossa, forming the perineal artery supplying anterior part of the perineum. In this region, perineal artery splits into the posterior scrotal artery and the transverse perineal artery. The bilobed flaps used in the present study were planned from the inguinal and medial thighs, where the pudendal arteries terminate [Figure 1]. Thus, it is basically an axial flap fed from the perforator arteries of the external and internal pudendal arteries.

- Bilobed pudendal flap and vascular support (A) Scrotal debridement and testicular wound care were performed to prepare the participants for reconstruction following Fornier’s gangrene. (B) Open testicular wound was fully covered through reconstruction using bilobed flap. (C) Post-treatment view of the reconstruction site
Flap design and elevation
The surgical technique used in the present study requires sufficient debridement of the scrotal area and proper dissection of the pudendal artery-based bilobed pudendal flap planned from the right medial thigh. Although all study flaps were planned from the right medial thigh due to sufficient flap size, it is equally possible to plan the flap from the left medial thigh area as well. The technique adopted in the present study differs from the classical bilobed flap technique in that the former requires the planning of defect and reconstructive tissues on different planes. In addition, the flaps used in the present study can move in each of the three planes, whereas the classic bilobed flap rotates primarily in one plane.
Three-axial motion capacity and sufficient size of the type of flaps used in the study allowed the closure of the defects using one single flap for each case. Flap thickness can be increased up to the fascial layer, and larger flaps can be prepared as long as the vascular pedicle is retained.
In classical bilobed flap design, the angle between the flap and the defect is 45°. In the technique used in this study, however, Flap A is the flap reconstructing the main defect and Flap B is the flap reconstructing the donor site of Flap A. Flap A and Flap B are planned to have a 90° angle between each other.
Although it was possible to plan the maximum flap size as 15 cm × 24 cm for Flap A and as 10 cm × 18 cm for Flap B, the maximum Flap A size used in the study cases was 11 cm × 20 cm and Flap B size was 7 cm × 15 cm. Flap A was planned parallel (and 2 cm distal) to the inguinal line, whereas Flap B was planned on the long axis of the medial thigh, making a right angle to Flap A. It was important to design Flap B on the long axis of the medial thigh for easy closure of the donor site. Flaps were dissected suprafascial to obtain a thinner texture. Lymphatic tissues and pudendal perforator artery/arteries were dissected carefully to preserve blood flow and lymphatic drainage of the bilobed flaps. After dissected, Flap A was transposed into the main defect area and Flap B to the donor site of Flap A. Donor site of Flap B, on the other hand, was covered by primary suturation. The flap was adapted to the defect area, and a hemovac drain was placed in the scrotal area [Figure 2]. In all study patients, the bilobed flap was planned from the right medial thigh as the flap size was sufficient for each defect. Flap thinning procedure was applied in no case. The hemovac drain was removed on the third postoperative day. Flap A rotates on axial, coronal, and sagittal planes.
- Gradual recovery in the first year of the reconstruction process. A, Debridement procedure after fasciitis. B, Defect was totally reconstructed using a bilobed pudendal flap obtained from the right medial thigh. C, Scrotal skin has been observed to magnificently match and function 1 year after reconstruction
Procedure
The patients under study were inserted a urinary catheter (catheter or cystofix) before the first debridement. Combined serial debridements and antibiotherapy were administered to take fasciitis under control. Negative pressure wound therapy was performed to prepare the defective areas for surgical reconstruction. Reconstruction was planned at the fourth or fifth week, on average, after infection of the serial wound cultures was brought under control.
RESULTS
Well vascularization of the distal portion of the flap prevented the rise of the need to perform deepithelialization in any participant. The shortest discharge time was recorded to be 4 days and the longest discharge time to be 8 days (7 days on average). No early complications such as infection, bleeding, hematoma, and partial or complete flap loss were observed in any case.
On the fifth postoperative day, 2-cm separation was observed at one end of Flap A in one patient, which was then primarily closed (suturation). The shortest follow-up period was observed to be 7 months and the longest follow-up period to be 19 months [Table 1]. Long-term evaluation of the study patients revealed no scar contraction, urinary problem, erectile dysfunction or discomfort, or sensory loss, which proved achievement of the targeted results. No morbidity developed in the donor site of any study patients during the repair process of the testicular skin defects. Thus, no repair-related issues arose that affect patient comfort during the whole study period.
| Patients | Age (years) | Comorbid diseases | Postoperative stay (days) | Complication | Follow-up period (month) |
|---|---|---|---|---|---|
| 1 | 50 | DM | 4 | No | 12 |
| 2 | 58 | DM | 6 | No | 11 |
| 3 | 64 | DM + CAD | 8 | No | 7 |
| 4 | 64 | DM + HL + CAD | 8 | Dehiscence | 19 |
| 5 | 65 | DM | 7 | No | 8 |
| 6 | 67 | DM | 7 | No | 14 |
| 7 | 68 | DM + CAD | 8 | No | 11 |
| 8 | 68 | DM + CAD | 8 | No | 17 |
DM = Diabetes mellitus, CAD = coronary artery disease, HL = hyperlipidemia
DISCUSSION
Scrotal skin defects resulting from Fournier’s gangrene should be repaired using single-stage, fast, simple methods achieving minimal morbidity to retain patient’s testicular viability and avoid psychological adverse effects.
However, debridement of the tissues devitalized due to necrotizing fasciitis is really a complicated issue, as it results in wide tissue defects in the urogenital region. As discussed above, there are options varying from skin grafts to many flaps such as musculocutaneous flaps, local fasciocutaneous random flaps, medial circumflex femoral artery perforator flaps, directly sensory flaps, single-lobe pudendal flap, and many others.
The use of bilobed pudendal flaps (planned from the pudendal thigh skin—femoral proximal inner surface—offering best thickness, color, and structure for scrotal reconstruction) in eight patients for reconstruction of total scrotal skin defect caused by Fournier’s gangrene in 2016–2019 enabled the present study to show the advantages of bilobed pudendal flaps over other existing methods.
Thanks to its thin structure, regional proximity, and vascular origin; the bilobed pudendal flap used in this study produced no early complications such as infection, scar formation, traction, and partial or complete flap loss in any of the study cases.
Thick and bulky musculocutaneous flaps, anterolateral flaps, and medial circumflex femoral artery perforator flaps are also used in the repair of scrotal defects. However, the flap type used in the present study has some advantageous over the concerned flap types. Study flaps were thinner as well as being flexible and comfortable; thanks to their low fat content. None of the study flaps required further microsurgical procedure.
Unlike anterolateral thigh flaps, none of the study flaps resulted in traction of flap pedicle; thus, leg movements brought no disadvantage to reconstruction. In addition, the single-step study procedure prevented the development of interstitial oedema, a complication observed to reduce pliability and functionality in the cases to have been applied perforator flaps and free flaps. However, “bilobed” study flaps enabled the prevention of pliability even from the minimal changes in the lymphatic flow.
As there is no reporting of contracture complaint by any participant, study flaps proved to be more successful in covering the large-scale defects of the study participants than the flaps planned from the opposite scrotum as well as the directly sensory flaps. In addition, sense was received directly from the pudendal nerve branches in the study patients. Physical examination proved that none of the study patients experienced any sensory problem related to the sense of touch and the feeling of hot/cold and pain. Besides, defect size-based planning capacity of the bilobed pudendal flaps enables successful reconstruction in difficult cases also.
The study flap produced successful results in the repair of scrotum (which does not provide sufficient amount of healthy tissue fore widening) severely infected by the Fournier’s Gangrene. The study flap was applied in one procedure (i.e., a one-stage flap). From this aspect, it is superior to the two-stage reconstruction using tissue expanders.
Although it offers many advantages for the patient and the surgical team over other methods, the use of a bilobed pudendal flap in the highly sensitive area of the scrotum requires experience. Fed from the perforator arteries of the external and internal pudendal arteries (thus, it is a great advantage to be able to evaluate vascular support of the flap before the surgery), the bilobed pudendal flap can be used to reconstruct scrotal skin defects when the vascular support is not affected by the debridement and gangrenous changes.
In addition, considering the facts that the vascular supply of the study flap is close to the trauma area and that the serial and aggressive defect debridements due to Fournier’s gangrene may result in the damage/sacrifice of the perforators supplying the study flap, sufficient blood flow may not be ensured. For this reason, study flaps may not be successful in cases when there are concerns about sufficient blood circulation. Therefore, this type of flap is not suggested for use in the absence of sufficient vascular supply and structures.
Although no thermoregulation test was performed on any study patient, because thin skin flaps are better in retaining thermoregulation function and considering that bilobed pudendal flap and scrotal skin are similar in terms of thickness, the bilobed pudendal flap was assumed to preserve testicular thermoregulation. The mean age of the study patients was high, and, as previously mentioned, each patient had comorbidity, which prevented the collection of a sufficient amount of test samples and standardization of results. For this reason, testicular functions of the study participants could not be evaluated.
Thus, one of the limitations of the study can be listed as high mean age. Therefore, the present study suggests further studies on younger populations to evaluate testicular functions.
In conclusion, in the light of the reliable, sensorial, and aesthetic results it produced, this study suggests pudendal artery-based bilobed pudendal flap as a rational option for scrotal skin reconstruction in appropriate patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Perineal reconstruction using a bilobed pudendal artery perforator flap. Gynecol Oncol. 2010;118:313-6.
- [Google Scholar]
- Reconstruction of vulva using pudendal thigh gluteal fold bilobed flap. J Plast Reconstr Aesthet Surg. 2010;63:e130-2.
- [Google Scholar]
- Scrotal reconstruction following Fournier gangrene using the medial circumflex femoral artery perforator flap. Ann Plast Surg. 2006;57:333-5.
- [Google Scholar]
- Reconstruction of wide scrotal defect using superthin groin flap. Urology. 2006;68:419-22.
- [Google Scholar]
- Superiority of medial circumflex femoral artery perforator flap in scrotal reconstruction. Ann Plast Surg. 2011;67:526-30.
- [Google Scholar]
- Fournier’s gangrene: A review of 43 reconstructive cases. Plast Reconstr Surg. 2007;119:175-84.
- [Google Scholar]
- One-stage reconstruction of the scrotum following Fournier’s syndrome using a probable arterial flap. Plast Reconstr Surg. 1980;66:608-12.
- [Google Scholar]
- Scrotal reconstruction after necrotizing cellulitis of the perineum and external genital organs. Apropos of 21 cases. Ann Urol (Paris). 1995;29:308-12.
- [Google Scholar]
- Reconstruction of the scrotum by inguinal flap after Fournier’s gangrene. Progres En Urologie. 2003;13:703-6.
- [Google Scholar]
- Penoscrotal reconstruction with superficial circumflex iliac artery perforator propeller flap. Microsurgery. 2019;39:688-95.
- [Google Scholar]
- Scrotal reconstruction using a superficial circumflex iliac artery perforator flap following Fournier’s gangrene. Int Wound J. 2016;13:996-9.
- [Google Scholar]
- A technique for improving cosmesis after primary scrotum reconstruction with skin grafts. Ann Plast Surg. 2015;75:205-7.
- [Google Scholar]
- Primary lymphedema of the scrotum: Surgical treatment and reconstruction. Ann Plast Surg. 1988;21:354-7.
- [Google Scholar]
- Scrotal reconstruction with modified pudendal thigh flaps. J Plast Reconstr Aesthet Surg. 2016;69:278-83.
- [Google Scholar]
- Use of tissue expansion for scrotal sac reconstruction after scrotal skin loss. Urology. 2005;65:1216-8.
- [Google Scholar]
- Reconstruction of scrotum with a free flap. Late results. Handchir Mikrochir Plast Chir. 1983;15:261-4.
- [Google Scholar]
- Meshed unexpanded split-thickness skin grafting for reconstruction of penile skin loss. J Urol. 2004;172:976-9.
- [Google Scholar]
- Utilities of split-thickness skin grafting for male genital reconstruction. Urology. 2015;86:835-9.
- [Google Scholar]
- Scrotal reconstruction with a rectus abdominis muscle flap. Br J Plast Surg. 1988;41:190-3.
- [Google Scholar]
- A new technique in scrotal reconstruction: Short gracilis flap. Urology. 2003;61:1254-6.
- [Google Scholar]
- An experiment study and clinical observation of the testicle spermatogenesis after scrotum reconstruction. Zhonghua Zheng Xing Wai Ke Za Zhi. 2004;20:203-5.
- [Google Scholar]
- A new repair technique for penile paraffinoma: Bilateral scrotal flaps. Ann Plast Surg. 1996;37:386-93.
- [Google Scholar]
- Scrotum reconstruction with neurovascular pedicled pudendal thigh flaps. Urology. 2007;70:170-2.
- [Google Scholar]
- The internal pudendal artery perforator thigh flap: A new freestyle pedicle flap for the ischial region. Plast Reconstr Surg Glob Open. 2014;2:e142.
- [Google Scholar]






