Translate this page into:
Efficacy of botulinum toxin type A injections in improving hypertrophic scarring and keloid formation: A systematic review and meta-analysis of randomized controlled trials
*Corresponding author: Hatan Mortada, Division of Plastic Surgery, Department of Surgery, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia. hatanmortada@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mortada H, Alowais FA, Alassaf H, Al Jabbar I, Albalawi IA, Alshomer F, et al. Efficacy of botulinum toxin type A injections in improving hypertrophic scarring and keloid formation: A systematic review and meta-analysis of randomized controlled trials. J Cutan Aesthet Surg. doi: 10.25259/jcas_34_2024
Abstract
Introduction:
This article aims to provide a systematic review of the use of botulinum toxin type A (BTA) in the prevention and treatment of hypertrophic scars and keloids. These types of scars pose significant challenges in clinical practice, and alternative treatment approaches are being explored. BTA has shown promise in its potential to modulate scar formation and improve outcomes.
Material and Methods:
Following the guidelines set forth by the preferred reporting items for systematic reviews and meta-analyses, a thorough examination of the available literature was conducted, encompassing the period from the inception of relevant databases until September 2023. The electronic databases utilized for this review included CENTRAL, MEDLINE, Google Scholar, and EMBASE.
Results:
Our review evaluated 1001 articles, ultimately including 12 randomized controlled trials that fulfilled our inclusion criteria. The visual analog scale (VAS) scores revealed a significant improvement in the cosmetic outcomes for the BTA group (mean difference [MD] 1.03, 95% confidence interval [CI] 0.01–2.05, P < 0.0001). Similarly, the vancouver scar scale (VSS) scores indicated superior scar quality in the BTA group (MD = −1.18, 95% CI −1.94 to −0.42, P = 0.001). Adverse events were minimal and included instances such as mild eyelid drooping and the development of an abscess requiring surgical intervention.
Conclusion:
Our systematic review and meta-analysis indicate that BTA significantly improves hypertrophic scars and keloids, as shown by better VAS and VSS scores. Adverse events were minimal. Further large-scale studies are needed for validation.
Keywords
Botulinum toxin type A
Hypertrophic scars
Keloids
Scar prevention
Scar treatment
Scar management
Scar modulation
Fibroblast proliferation
Collagen synthesis
Scar hypertrophy
INTRODUCTION
Scar tissue formation is a natural part of the healing process following skin injury or surgical procedures. However, certain individuals are more susceptible to developing hypertrophic scars and keloids. Hypertrophic scars are characterized by excessive fibrosis confined to the injury site, which tends to regress over time. On the other hand, keloids involve excessive fibrosis that extends beyond the injury area and does not regress. Severe cases of hypertrophic scars and keloids can even affect joints and the mouth, significantly impacting the quality of life for those affected. Traditional treatment approaches for hypertrophic scars and keloids encompass massage therapy, silicone gel treatment, laser therapy, light therapy, and radiotherapy.1 Moreover, emerging treatment options include intralesional cryotherapy, as well as intralesional injections of 5-fluorouracil, interferon, corticosteroids, and bleomycin.2
Treating hypertrophic scars and keloids poses challenges and complexities. Before initiating treatment, it is crucial to conduct a comprehensive evaluation of the lesion, taking into account factors such as size, location, and any associated pain or tenderness. In addition, understanding each patient’s treatment expectations and adopting a multidisciplinary therapeutic approach are essential.3-5 Despite various treatment options available for hypertrophic scars and keloids, there is currently no universally accepted standard of care, and management decisions are often based on individual clinical experience.6
Intralesional corticosteroid injections are commonly utilized; however, they can lead to complications, including pain and itching following the injections.7 As a result, recent studies have explored the use of botulinum toxin type A (BTA) injections to inhibit the formation of hypertrophic scars and keloids, offering the advantage of reduced discomfort and adverse events.8,9 Nonetheless, the routine use of intralesional BTA in clinical practice remains limited, and there is a scarcity of multicenter clinical trials with a substantial number of participants to establish robust evidence supporting its efficacy in the treatment of hypertrophic scars and keloids. Previous research has examined the effectiveness of intralesional BTA injections in the treatment of hypertrophic scars and keloids. One systematic review and meta-analysis conducted by Bi et al. in 201910 concluded that BTA was more effective than placebo or intralesional corticosteroid injections. Another study by Wang et al.11 specifically focused on facial scars and demonstrated that botulinum toxin improved their appearance with acceptable safety outcomes. However, many of the existing systematic reviews on this topic have notable limitations. These limitations often include inadequate predefined search strategies, failure to adhere to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines for systematic reviews, and insufficient assessment of bias in the primary studies included in the study. Therefore, the primary goal of this systematic review and meta-analysis was to comprehensively assess and compare the effectiveness of intralesional BTA injections versus placebo and intralesional corticosteroid injections versus placebo in the management of hypertrophic scars and keloids. In addition, this review aimed to address the limitations of previous studies by including up-to-date articles available, thus providing the most current evidence on the topic. By synthesizing the findings from these studies, this review aimed to offer valuable insights into the relative efficacy of these treatments and contribute to the existing body of knowledge in the field.
MATERIAL AND METHODS
Search strategy
This systematic review adhered to the guidelines outlined in the PRISMA.12,13 To ensure a thorough and comprehensive analysis, a meticulous literature search was performed from the inception of four prominent databases (CENTRAL, MEDLINE, Google Scholar, EMBASE, and Web of Science) until September 2023, with no restrictions on the timeframe. The search strategy employed a combination of relevant keywords to yield comprehensive results. The key terms used in the search included scar, botulinum toxin, botulinum, toxins, randomized controlled trials (RCTs), controlled clinical trials, hypertrophic scar, keloid, and split scar. By employing these specific search terms, the review aimed to encompass a wide range of relevant studies and provide an up-to-date and comprehensive analysis of the topic.
Study selection
Two authors utilized the Rayyan collaboration platform for the initial screening of articles based on title and abstract.14 Subsequently, the full texts of all potentially relevant studies were reviewed. In cases where disagreements arose, a senior third reviewer was involved to reach a consensus. Only studies that met the predefined inclusion and exclusion criteria were included in the analysis. The review focuses on studies that examined the effects of BTA administered between 6 and 14 days post-surgery, which is the most commonly reported timeframe in the literature. Table 1 provides an overview of our study’s inclusion and exclusion criteria, which were established based on the population, intervention, comparison, and outcome framework.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Study design | Randomized controlled trials | Case reports, reviews, animal studies, prospective and retrospective cohort studies, commentaries, cross-sectional studies, and case series. |
| Population | Patients with hypertrophic scars or keloids | Patients without hypertrophic scars or keloids |
| Intervention | Use of Botulinum Toxin Type A injections | Other forms of treatment such as laser and surgery. |
| Outcome measures | Scar width, height, color, texture, and patient-reported outcomes | Studies that do not measure relevant outcomes |
| Language | Published in English | Published in languages other than English |
Data extraction
The process of data extraction was conducted independently by two authors, who extracted relevant information from the text, tables, and figures of the included studies using a standardized extraction form that had been pre-designed. To ensure the reliability and accuracy of the extracted data, a second author performed an independent review of the data extraction process. This involved cross-checking all extracted data points against the source materials to identify any discrepancies or missing information. The extracted data encompassed various essential elements, including study characteristics (such as author, year of publication, study design, country of origin, and sample size), participant characteristics (including duration of follow-up, number of patients, treatment details including use and dose, location of the scar, and outcome indicators), and the type of statistical analysis used to evaluate study outcomes. In cases where the extracted data were unclear or incomplete, efforts were made to contact the corresponding authors of the respective studies to seek clarification. If missing data could not be obtained, a comprehensive explanation was provided regarding the missing data and its potential impact on the reported results. The management of the extracted data was overseen by the first author, in consultation with the second author, to ensure proper organization and accuracy throughout the process.
Bias and quality assessment
Two authors conducted independent assessments of the quality of evidence for the outcomes using the GRADEpro Guideline Development Tool.15 The risk of bias (RoB) in the included studies was evaluated by considering factors such as random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias.16 The same two authors also independently evaluated the studies for five specific sources of bias, including the generation of allocation sequence, allocation concealment, investigator blindness, description of withdrawals and drop-outs, and the efficacy of randomization. In cases where disagreements occurred, they were resolved by involving a senior author. The assessment of bias risk in the RCTs was carried out by two reviewers independently using the Cochrane risk-of-bias tool. This tool allowed for the evaluation of various factors, including randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, handling of incomplete data, and selective reporting. Each study category was rated based on these criteria to determine the level of bias present in the trials.
Statistical analysis
Statistical analysis was conducted using RevMan software (version 5.4, the Cochrane Collaboration, 2020). Continuous data were compared using mean difference (MD) or standardized MD with a 95% confidence interval (CI), while dichotomous data were compared using risk ratios with a 95% CI. The heterogeneity among the included studies was assessed using I2, with values ranging from 0% to 40% indicating unimportant heterogeneity, 30–60% indicating moderate heterogeneity, 50–90% indicating substantial heterogeneity, and 75–100% indicating considerable heterogeneity. A fixed-effect model was employed when I2 was <50% and a random-effects model was used otherwise. Statistical significance was determined based on P < 0.05.
RESULTS
Review of the included studies
The systematic review and meta-analysis initially identified 1724 citations through various databases. Specifically, 350 citations were from PubMed, 810 from Embase, 95 from Cochrane, 269 from Web of Science, and 200 from Google Scholar. After removing duplicate citations, a total of 1001 studies remained for further evaluation. The screening process involved assessing the titles and abstracts of these studies, resulting in 41 articles that were reviewed in full text. Finally, after careful evaluation, only 12 articles met the inclusion criteria and were included in the final review. These articles are referenced as.17-28 The exclusion of articles and the entire screening process, following the guidelines outlined in the PRISMA, are visually represented in Figure 1.
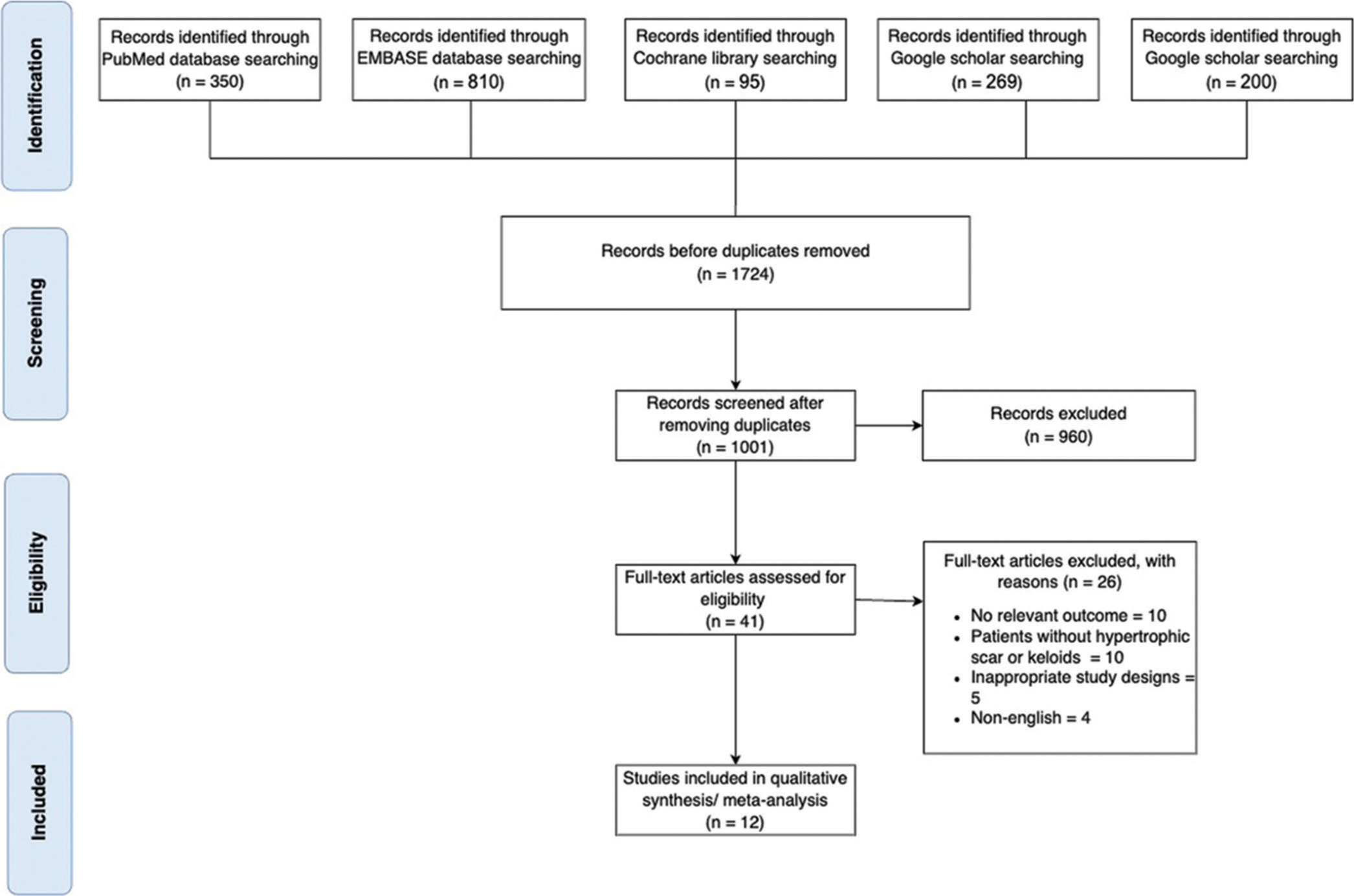
- Preferred reporting items for systematic reviews and meta-analyses flowchart.
Characteristics of the included studies
The included studies were published between 2014 and 2023. In the systematic review of 12 studies, all were RCTs. The country of origin varied: China and Korea were represented most frequently with three studies each, followed by Taiwan and Egypt with two studies each. Switzerland and Syria were each represented by a single study. In the BTA group, the sample sizes ranged from 14 to 30 participants, with a total of 263 patients. This suggests that there are multiple studies within the BTA group, each with different sample sizes. The total number of patients in the BTA group is 263 patients.
On the other hand, the control groups’ sample sizes ranged from 12 to 30 patients, with a total sample size of 260 patients. Similar to the BTA group, there are likely multiple studies within the control group, each with varying sample sizes. The total number of patients in the control group is 260. Table 2 presents the detailed characteristics of the included studies, including information about the interventions in each group, age, gender, and other relevant characteristics. The review revealed scars from varied etiologies: 16 from cardiac surgeries, including 11 from valve replacements; two from cleft lip repairs; one from a canthal area procedure; two from thyroidectomies; 15 from burns (ten fire-related, five from boiled water); 30 from trauma, notably 11 from blunt force and ten from slips; and one from facial surgery through a submandibular approach. The review identified different scar types: Eight cases of keloids and 27 cases of hypertrophic scars. Some studies reported a combination of hypertrophic scars and keloids.
Regarding the timing of injections, several studies administered treatment immediately after wound closure. The average injection times varied: one study reported 9.1 days post-surgery (range 7–12 days), another at 6.4 days after epicanthoplasty (range, 6–7 days), and yet another at 6.6 days post-surgery (range 5–9 days). Some studies exhibited a broader range, with injections occurring anywhere from immediately after skin closure up to 14 days. In addition, one study opted for treatment within 5 days of primary closure. Scar lengths from the studies varied widely. Two studies provided measurements for different groups: in one, Group 1 had an average scar length of 0.69 ± 0.24 cm, and Group 2 had 0.61 ± 0.25 cm, while another study showed the treatment group at an average of 0.78 ± 0.38 cm and the control group at 0.94 ± 0.77 cm. Specific lengths from other studies included 8 cm (ranging from 5 to 15 cm), 6.74 cm (ranging from 3 to 16 cm), 4 cm, and 8.64 cm (ranging from 5 to 15 cm). Table 3 depicts the location of scars on the included patients. The doses of botulinum neurotoxin type A (BoNT-A) administered across the studies varied, with an average dose of approximately 39.71 U. The overall range of doses used across the studies spanned from 5 U to 100 U.
| Study ID | Study design | Country | Total number of patients | Age (Mean, ± SD) | Clinical recommendations | Level of evidence | |
|---|---|---|---|---|---|---|---|
| Shaarawy et al. 201417 | Randomized Controlled Trial | Egypt | 24 | 29.29±11.793 y | • Corticosteroid therapy: It’s recommended to repeat every 4 weeks for six sessions or until the keloid has improved. • BTA: Administer at a concentration of 5 IU/cm3and repeat every 8 weeks for three sessions or until improvement is noted. • Given its effectiveness, the higher cost of BTA is a limiting factor for its routine use. However, integrating BTA with corticosteroids could counteract the potential drawbacks of both treatments. |
II | |
| Li et al. 201818 | Randomized Controlled Trial | China | 17 | Range: 25–62 y | • The authors posited that BTA’s tension-reducing properties, combined with its antagonistic effect on fibroblast differentiation and TGF-b1 expression, indicate its potential to prevent hypertrophic scar development in median sternotomy wounds. • The authors pointed out that the timing of the BTA injection might be crucial. In their study, BTA was injected approximately 9.1 days after surgery. They acknowledge that this post-surgery interval might influence the effectiveness of BTA in scar management. |
II | |
| Chang et al. 201419 | Randomized Controlled Trial | Taiwan | 60 | Study Group Control Group |
3.17±0.25 mo 3.13±0.37 mo |
• The author suggests that while their established method of repair and taping controls wound tension effectively, BTA offers an additional benefit to scar appearance, especially in reducing scar width. • No complications were reported due to the BTA injections. Previous research indicates that BTA is safe for use in children younger than 2 years, especially in dosages lower than those used for conditions like obstetric brachial plexus palsy and cerebral palsy. |
I |
| Chang et al. 201420 | Double-Blinded, Vehicle- Controlled Randomized Controlled Trial | Taiwan | 60 | Study Group Control Group |
24.70±7.16 y 21.87±8.00 y |
• The study concludes that BTA significantly improved the quality of scarring following CLSR. The recommendation stemming from this research is that botulinum toxin injections, due to their paralyzing effect, can be beneficial in reducing tension around healing facial wounds, subsequently leading to better scar outcomes. | I |
| Huang et al. 201921 | Double-Blinded, Split-Face Randomized Controlled Trial | Switzerland | 43 | Average 23.6 y; Range: 20–39 y | • Early postoperative administration of BTA in the medial canthal region effectively reduces hypertrophic scarring and improves the outcome of epicanthoplasty. • During injections in the medial canthal region, use precise subcutaneous techniques to prevent complications like eyelid drooping. In the study, one participant experienced mild lid drooping, which self-resolved without intervention. |
II | |
| Kim et al. 201422 | Double-Blinded, Split-Scar Randomized Controlled Trial | Korea | 15 | Average 46 y; Range: 31–60 y | • The timing of the BTA injection is crucial. While this study injected BTA at an average of 6.6 days post-surgery, it’s suggested that BTA might have more pronounced benefits when administered either just before wound closure or right after it, during the very early stages of wound healing. • The author recommends future studies that compare different time points for BTA injection (e.g., before surgery, right after wound closure, or during early follow-up visits) to determine the most effective treatment protocol for surgical scars. |
II | |
| Hu et al. 201723 | Prospective, Double-Blinded, Split-Scar Randomized Controlled Trial | China | 14 | Average 12 y; Range: 6–49 y | • The authors note that while some research injected BTA several days post-surgery, they believe that BTA might be more effective when administered immediately after wound closure. This suggests that the timing of the BTA injection is critical to its effectiveness. | II | |
| Tawfik et al. 202324 | Intra-Patient Randomized Controlled Trial | Egypt | 15 | 7.2±4.2 y | • The BTA enhanced the appearance of scars by improving their pliability, erythema, and thickness. Notably, there was significant improvement in the vascularity and pliability of the scars. Additionally, the mobility of joints previously restricted by hypertrophic lesions showed dramatic improvement. | II | |
| Alrmeela et al. 202225 | Split-Scar Randomized Controlled Trial | Syria | 15 | 33.27±14.82 y | • The optimal time for BTA injection is recommended to be the 7thpostoperative day based on the duration of the inflammatory phase of wound healing and previous studies' findings. • The authors emphasize the utility of the VAS for assessing simple facial wound scars due to its sensitivity, ease of use, and reproducibility. • BTA is recommended because it relieves tension on wound edges (by causing temporary muscle paralysis), reduces collagen deposition, and directly inhibits fibroblast proliferation and differentiation. The authors believe these properties make BTA effective in preventing scars after a submandibular approach. |
II | |
| Chen et al. 2021 26 | Prospective, Double-Blinded, Split-Scar Randomized Controlled Trial | China | 20 | Average 37 y; Range: 18–52 y | • For non-melanoma skin cancer patients, BTA can be applied after surgical treatment, especially for those who are more concerned about postoperative scars. • A high-dose BTA injection immediately after the surgical procedure is recommended to achieve better scar beautification effects, as it was found to be more effective than the low dose in managing scar hypertrophy. |
II | |
| Lee et al. 201727 | Randomized Controlled Trial | Korea | 30 | Study Group | 34.33 y; Range: 18–69 y | • BTA might be more beneficial if administered earlier in the wound-healing process. Reconstituting BTA with 1% lidocaine with 1:100,000 epinephrine might lead to instant paralysis, offering potential benefits over the delayed action of BTA in 0.9% saline. • The effects of BTA on scar maturation might not be immediately visible within the 1stmonth but may show after 6 months. |
II |
| Control Group | 30.27 y; Range: 18–53 y | ||||||
| Bae et al. 202028 | Prospective, Double-Blinded Randomized Controlled Trial | Korea | 40 | Study Group | 50.20±9.51 y; Range: 29–67 y | • Surgical residents should be trained not only in surgical techniques but also in how to prevent or minimize scar formation during wound closure. • Consider injecting BTA directly into the muscle just before skin closure during surgery for a more immediate and individualized assessment of the wound. |
II |
| Control Group | 50.50±8.88 y; Range: 31–67 y |
Mo: Months, Y: Years, BTA: Botulinum Toxin type A, TGF-b: Transforming growth factor beta. CLSR: Cleft lip scar revision, VAS: Visual analog scale
| Site | Number | Percentage |
|---|---|---|
| Face | ||
| Canthal | 30 | 9.8 |
| Upper lip | 120 | 39.2 |
| Forehead | 30 | 9.8 |
| Other | 73 | 23.9 |
| Trunk | ||
| Chest | 18 | 5.9 |
| Back | 3 | 0.98 |
| Abdomen | 3 | 0.98 |
| Extremity | 5 | 1.6 |
| Other sites | 24 | 7.8 |
Visual analog scale (VAS) score
The visual analog scale (VAS) score is a widely used scale for evaluating cosmetic outcomes of the skin, ranging from worst (0 points) to best (10 points). Five studies, encompassing 215 patients, provided data on the VAS score. The results indicate that the VAS score in the BTA group was significantly higher than in the control group (MD 1.03, 95% CI 0.01–2.05, P < 0.0001), although with high heterogeneity, I2 = 93%, P = 0.05, as shown in Figure 2.
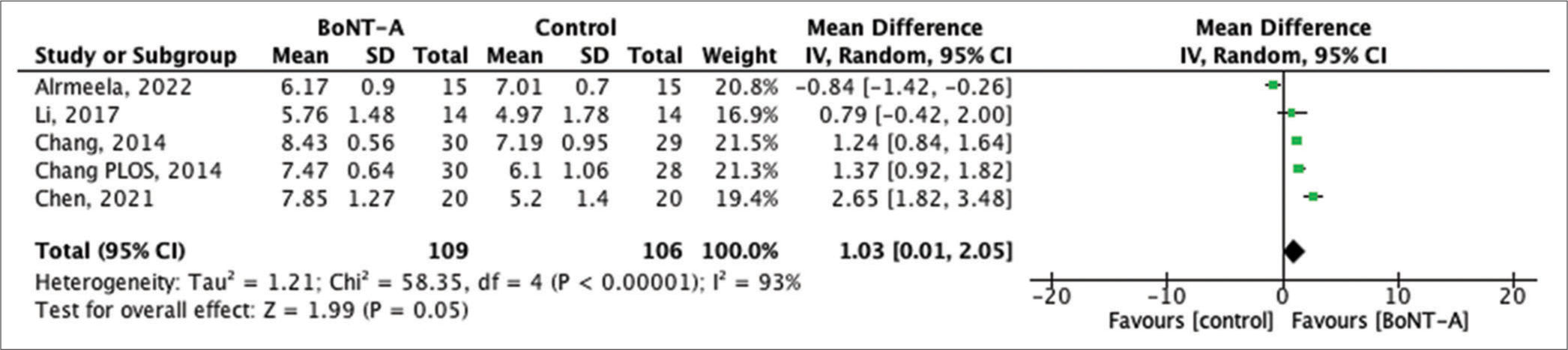
- Forest plot of the pooled studies showing visual analog scale score comparisons between botulinum toxin type A and control treatments in scar management. SD: Standard deviation, CI: Confidence interval, BoNT- A: Botulinum neurotoxin type A.
Vancouver scar scale (VSS) score
The vancouver scar scale (VSS) score, specifically crafted for evaluating scars, comprises four components: scar height (0–4 points), vascularity (0–3 points), pigmentation (0–3 points), and pliability (0–5 points). Higher scores correlate with more pronounced scarring. Data from seven RCTs detailed the VSS scores. The findings revealed a notable difference between the BTA group and the control group (MD = −1.18, 95% CI −1.94 to −0.42, P = 0.001), indicating that scars treated with BTA injections were of superior quality compared to those in the control group [Figure 3].
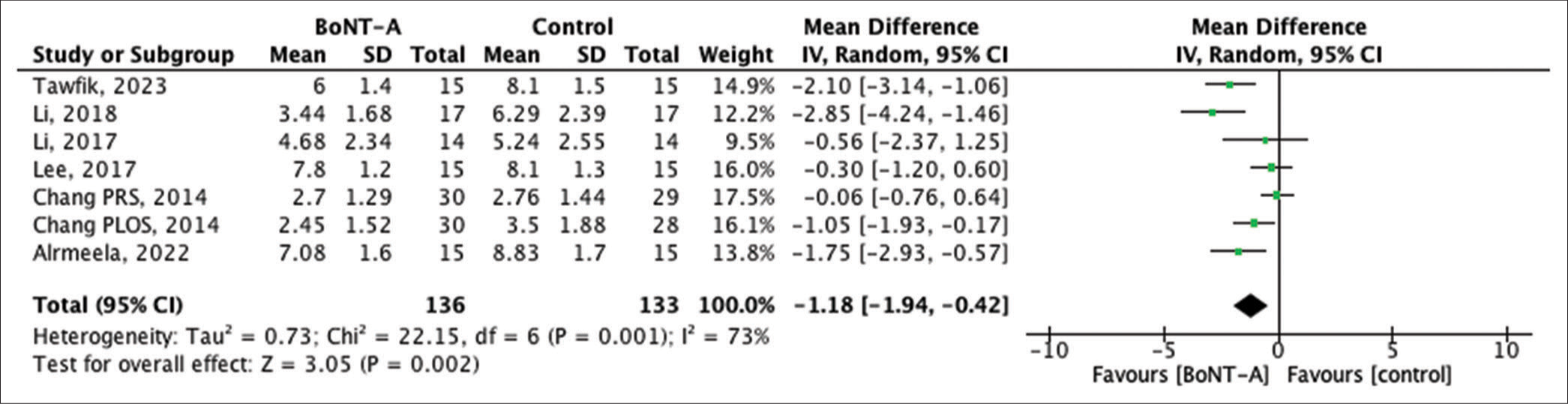
- Forest plot of the pooled studies showing Vancouver scar scale score comparisons between botulinum toxin type A and control treatments in scar management. SD: Standard deviation, CI: Confidence interval, BoNT-A: Botulinum neurotoxin type A.
Scar width
The width of a scar signifies the spread and growth of the scar post-wound healing. Four RCTs presented data on scar width following BTA treatment. The results suggested a slight improvement in scar width within the BTA group, although this difference was not statistically significant, MD = −0.03, 95% CI −0.27–0.22, P = 0.83, as depicted in Figure 4.
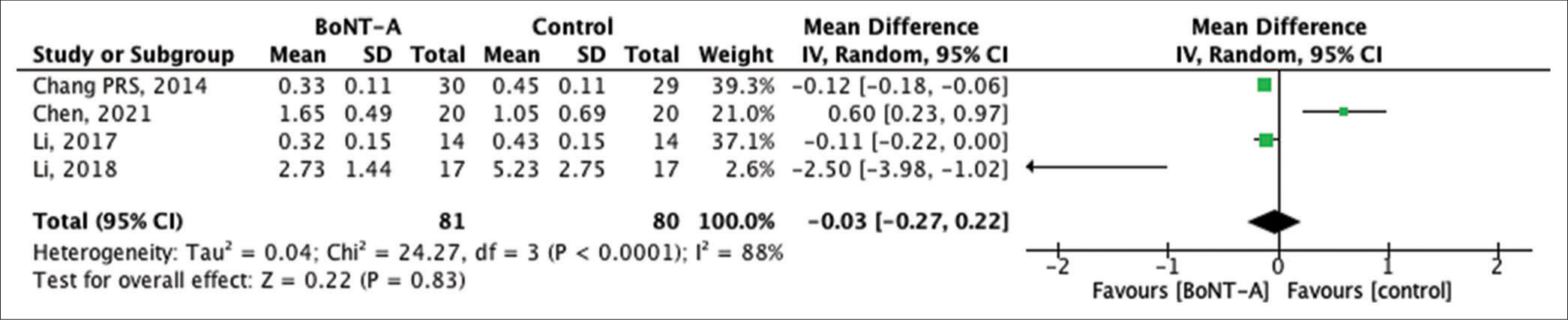
- Forest plot of the pooled studies showing scar width comparisons between botulinum toxin type A and control treatments. SD: Standard deviation, CI: Confidence interval, BoNT-A: Botulinum neurotoxin type A.
Adverse events
Complications following BoNT-A injections were documented in two studies. These included a case of mild drooping of the eyelid and another instance where a patient developed an abscess that necessitated surgical revision.
Publication bias and quality assessment of the included studies
Given the limited number of studies included in this analysis, Egger’s test was employed to identify any potential publication bias. The results of the test did not indicate significant publication bias (P = 0.812).
Quality assessment and bias evaluation
Two independent reviewers utilized the Cochrane RoB Assessment Tool for Randomized Trials (RoB 2) to assess the RoB in the eligible RCTs.16 This tool was employed to identify and evaluate potential sources of bias within the included RCTs. The findings indicated that one study had some concerns about the RoB, while the remaining studies demonstrated a low RoB as determined by the Revised Cochrane Tool [Figure 5].
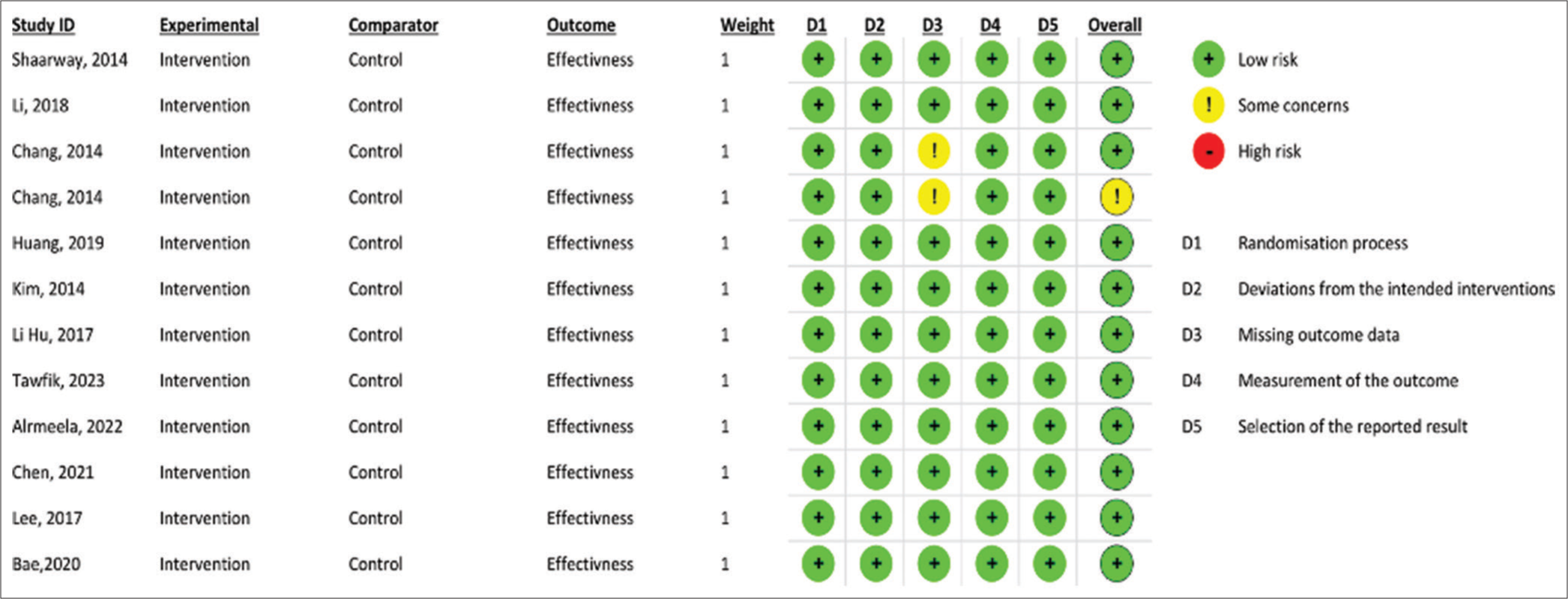
- Bias assessment of the included studies.
DISCUSSION
Our meta-analysis integrated findings from 12 studies, including 1060 patients. We focused on evaluating the therapeutic efficacy of BTA for managing hypertrophic scars and keloids. The results underscore BTA’s potential to not only alleviate scar hypertrophy but also to markedly improve scar esthetics, partly by inhibiting fibroblast proliferation and collagen production.
A scar is the final outcome of the wound-healing process and is an unavoidable consequence of surgical intervention. Hypertrophic scars and keloids pose problems for plastic surgeons globally due to their functional and cosmetic impacts, which can affect social interactions and overall quality of life.29 To reduce the development of a distinct scar, common procedures to support beneficial healing consist of using less reactive suture material, achieving a high-quality closure, applying occlusive or semi-occlusive dressings, and avoiding sun exposure.14 Recently, the use of BTA injections to decrease the edge of hypertrophic scars and keloids, the first split-scar, double-blind, and RCTs examining BTA for wound treatment was documented.30 The precise process through which BTA influences scar development remains unclear, and its use in preventing scars is not yet widely recognized in current medical practices. Recently, studies have shown that BTA can slow down the growth of fibroblasts and lower the levels of transforming growth factor beta-1.31,32 Moreover, BTA appears to stop fibroblasts from turning into myofibroblasts. This suggests that it could be beneficial in treating wounds that might result in raised scars after surgery.33
Our findings indicated that scars treated with BTA injections exhibited more favorable outcomes than those not treated with BTA. Specifically, the cosmetic appearance of scars, as measured by the VAS score, was significantly enhanced in the BTA group compared to the control group. Furthermore, the VSS score used for evaluating scars suggested that BTA-treated scars were of superior quality. However, there was only a minor improvement in scar width in the BTA group, and this difference was not statistically significant. Some adverse events, such as eyelid drooping and abscess formation post-BTA injection, were noted, but the overall treatment was deemed effective in improving scar quality. The results align with those from earlier studies and literature reviews. Research by Liu et al.34 and Fanous et al.35 demonstrated that BTA reduced hypertrophic scars and keloids in animal experiments. BTA shows potential as an effective treatment for hypertrophic scars and keloids; however, its benefits should be carefully weighed against established options like corticosteroid injections, laser therapy, and cryotherapy. Every technique comes with its benefits, potential side effects, and effectiveness. For instance, while corticosteroid injections have been excellent treatment of scars, they sometimes come with side effects such as skin thinning and telangiectasia (Visible blood vessels).36 On the other hand, the use of BTA for hypertrophic scars and keloids needs more in-depth studies on the effects of BTA on pathologic scars and/or mature keloids are needed before a comparatively expensive therapy for this particular indication can be postulated.
The timing of BTA injections can significantly influence the therapeutic outcomes for hypertrophic scars and keloids. Early intervention may allow for modulating the initial inflammatory response, which is critical for the formation and progression of these pathological scars. Indeed, Gassner et al., showed that injecting botulinum toxin into the muscles near the wound using 15 U of BTA for every 2 cm of surgical length within a day after closing the wound led to improved wound healing and scars that were less visible than those treated with a placebo.37 The majority of earlier research indicated that BTA was administered either immediately before or just after suturing the wound to inhibit scar development.38-40
While BTA has shown promising results in the treatment of hypertrophic scars, its efficacy in keloid management is equally important to consider. Hypertrophic scars, characterized by excessive but localized fibrosis, generally regress over time. In contrast, keloids involve aggressive fibrosis that extends beyond the original injury site and does not regress spontaneously. The studies included in our analysis indicate that BTA effectively reduces scar hypertrophy and improves esthetic outcomes in both scar types. However, keloids, due to their more persistent and invasive nature, may require a different therapeutic approach or combination of treatments for optimal outcomes. Future research should further investigate the specific mechanisms by which BTA influences keloid formation, as well as its long-term efficacy in treating this challenging condition. While BTA presents an exciting frontier in scar management, particularly for hypertrophic scars and keloids, it is essential to consider the broader picture. The treatment, while innovative, can be relatively more expensive compared to other more established modalities. This might hinder its widespread acceptance, especially in settings where healthcare resources are limited. However, with the promising results we have seen, there could be a shift toward this treatment if further studies can justify the cost with long-term benefits. In addition, considerations specific to the patient, like the type of scar, their unique healing tendencies, and possible allergic responses to treatments, should be integrated into the treatment selection process.
The relative novelty of BTA in this application means we are still learning about its full range of effects and potential complications. For patients and practitioners alike, this means proceeding with an informed perspective, taking into account both the promising potentials and the inherent uncertainties. Finally, while our meta-analysis sheds light on the potential of BTA, we still need more studies to have full knowledge about the full range of effectiveness of BTA.
CONCLUSION
Our comprehensive meta-analysis indicates that BTA holds significant promise in managing both hypertrophic scars and keloids. The data demonstrate BTA’s ability to reduce scar hypertrophy and improve esthetic outcomes, as reflected by better VAS and VSS scores. However, its effect on scar width was not statistically significant. The minimal adverse events reported, such as mild eyelid drooping and abscess formation, emphasize the need for further long-term studies to assess the safety and sustained efficacy of BTA.
While BTA shows potential in treating both scar types, keloids – due to their more aggressive nature – may respond differently compared to hypertrophic scars, requiring tailored approaches. Future research should explore the timing, dosage, and mechanisms of BTA in both hypertrophic scars and keloids. Comparative studies with other treatments, like corticosteroids, will also help clarify BTA’s place in scar management. Finally, cost-benefit analyses will be crucial in assessing the practicality of integrating BTA into standard clinical protocols for both hypertrophic scars and keloids.
Authors’ contributions
Hatan Mortada: Conceptualization of the project, supervision, statistical analysis, manuscript drafting, and critical revision of the manuscript. Feras Alshomer: Supervision, methodology development, and critical review of the manuscript. Fahad Abdullah Alowais: Data collection, interpretation, and contribution to the writing and editing of the manuscript. Hala Alassaf: Data collection, analysis, and manuscript preparation. Imtinan Al Jabbar: Literature search, screening of studies, and data extraction. Ibrahim Abdullah S. Albalawi: Literature search, data extraction, and contribution to manuscript drafting. Bassam Alawirdhi: Manuscript review.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burns. 2014;40:1255-66.
- [CrossRef] [PubMed] [Google Scholar]
- Management of keloids and hypertrophic scars: Current and emerging options. Clin Cosmet Investig Dermatol. 2013;6:103-14.
- [CrossRef] [PubMed] [Google Scholar]
- German S2k guidelines for the therapy of pathological scars (hypertrophic scars and keloids) J Dtsch Dermatol Ges. 2012;10:747-62.
- [CrossRef] [PubMed] [Google Scholar]
- The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010;125:557-68.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertrophic scars and keloids--a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171-81.
- [CrossRef] [PubMed] [Google Scholar]
- Updated international clinical recommendations on scar management: Part 2--algorithms for scar prevention and treatment. Dermatol Surg. 2014;40:825-31.
- [Google Scholar]
- Efficacy of IPL device combined with intralesional corticosteroid injection for the treatment of keloids and hypertrophic scars with regards to the recovery of skin barrier function: A pilot study. J Dermatolog Treat. 2015;26:481-4.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin and burn induces contracture. Arch Plast Surg. 2016;43:609-11.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin A for the treatment of keloids. Skin Pharmacol Physiol. 2012;25:313-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional injection of botulinum toxin type A compared with intralesional injection of corticosteroid for the treatment of hypertrophic scar and keloid: A systematic review and meta-analysis. Med Sci Monit. 2019;25:2950-8.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of botulinum toxin type a injections in improving facial scars: A systematic review and meta-analysis. Pharmacology. 2022;107:241-9.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009;151:W65-94.
- [CrossRef] [PubMed] [Google Scholar]
- Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Open Med. 2009;3:e123-30.
- [CrossRef] [PubMed] [Google Scholar]
- Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
- [CrossRef] [PubMed] [Google Scholar]
- McMaster University. 2015. Available from: https://gradepro.org/cite [Last accessed on 2024 May 03]
- [Google Scholar]
- Assessing risk of bias in included studies In: Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions.Version 5.1.0., Ch. 8. 2011. Available from: https://www.handbook.cochrane.org [Last accessed on 2024 May 03]
- [Google Scholar]
- Intralesional botulinum toxin type A equally effective and better tolerated than intralesional steroid in the treatment of keloids: A randomized controlled trial. J Cosmet Dermatol. 2015;14:161-6.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, placebo-controlled, double-blind, prospective clinical trial of botulinum toxin type a in prevention of hypertrophic scar development in median sternotomy wound. Aesthetic Plast Surg. 2018;42:1364-9.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin to improve results in cleft lip repair. Plast Reconstr Surg. 2014;134:511-6.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin to improve results in cleft lip repair: A double-blinded, randomized, vehicle-controlled clinical trial. PLoS One. 2014;9:e115690.
- [CrossRef] [PubMed] [Google Scholar]
- Early postoperative application of botulinum toxin type a prevents hypertrophic scarring after epicanthoplasty: A split-face, double-blind, randomized trial. Plast Reconstr Surg. 2019;144:835-44.
- [CrossRef] [PubMed] [Google Scholar]
- Early postoperative treatment of thyroidectomy scars using botulinum toxin: A split-scar, double-blind randomized controlled trial. Wound Repair Regen. 2014;22:605-12.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of botulinum toxin on improving facial surgical scars: A prospective, split-scar, double-blind, randomized controlled trial. Plast Reconstr Surg. 2018;141:646-50.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of botulinum toxin type A for treating post burn hypertrophic scars and keloid in children: An intra-patient randomized controlled study. J Cosmet Dermatol. 2023;22:1256-60.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the efficacy of botulinum toxin type A injection on improving the submandibular approach scars: Randomized controlled trial, Split-Scar. Eval Split Scar. 2022;45
- [Google Scholar]
- The effect of botulinum toxin injection dose on the appearance of surgical scar. Sci Rep. 2021;11:13670.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of early postoperative botulinum toxin a injection for facial scars. Aesthetic Plast Surg. 2018;42:530-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of botulinum toxin a on scar healing after thyroidectomy: A prospective double-blind randomized controlled trial. J Clin Med. 2020;9:868.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertrophic scarring: The greatest unmet challenge after burn injury. Lancet. 2016;388:1427-36.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of UV irradiation on cutaneous cicatrices: A randomized, controlled trial with clinical, skin reflectance, histological, immunohistochemical and biochemical evaluations. Acta Derm Venereol. 2007;87:27-32.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of botulinum toxin type A on transforming growth factor beta1 in fibroblasts derived from hypertrophic scar: A preliminary report. Aesthetic Plast Surg. 2010;34:424-7.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin type a inhibits connective tissue growth factor expression in fibroblasts derived from hypertrophic scar. Aesthetic Plast Surg. 2011;35:802-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of botulinum toxin type a on differentiation of fibroblasts derived from scar tissue. Plast Reconstr Surg. 2015;136:171e-8e.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of BTAA on inhibiting hypertrophic scar formation in a rabbit ear model. Aesthetic Plast Surg. 2017;41:721-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of keloid scars with botulinum toxin type A versus triamcinolone in an athymic nude mouse model. Plast Reconstr Surg. 2019;143:760-7.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin to improve facial wound healing: A prospective, blinded, placebo-controlled study. Mayo Clin Proc. 2006;81:1023-8.
- [CrossRef] [PubMed] [Google Scholar]
- Use of botulinum toxin type A to improve treatment of facial wounds: A prospective randomised study. J Plast Reconstr Aesthet Surg. 2013;66:209-14.
- [CrossRef] [PubMed] [Google Scholar]
- Donor site aesthetic enhancement with preoperative botulinum toxin in forehead flap nasal reconstruction. Ann Plast Surg. 2016;77:535-8.
- [CrossRef] [PubMed] [Google Scholar]
- Discussing fractional carbon dioxide laser and other physical treatments for scar prevention with patients. JAMA Dermatol. 2015;151:815-6.
- [CrossRef] [PubMed] [Google Scholar]







