Translate this page into:
Unravelling Masson’s tumor: A rare case of atypical presentation with comprehensive clinico-histopathological and radiological correlation
*Corresponding author: Amandeep Singh, Department of Radiodiology, Sri Guru Ram Das Institute of Medical Sciences and Research, Amritsar, Punjab, India. dr.amancs@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Awal G, Shubham, Parmar J, Singh A, Kaur N. Unravelling Masson’s tumor: A rare case of atypical presentation with comprehensive clinico-histopathological and radiological correlation. J Cutan Aesthet Surg. doi: 10.25259/JCAS_19_2025
Abstract
Masson’s tumor, or intravascular papillary endothelial hyperplasia (IPEH), is a rare, benign vascular lesion accounting for 2–4% of skin vascular tumors. Characterized by endothelial cell proliferation within an organized thrombus, it typically presents as a bluish-to-red, non-pulsatile nodule, often localized to the head, neck, and extremities. Rarely, it can occur at atypical sites such as the parotid gland, kidneys, and sinonasal cavity. This report describes a unique case of IPEH in a 37-year-old male with multiple, widely distributed nodules persisting for 4 years. The histopathological evaluation confirmed the diagnosis, revealing endothelial hyperplasia within vascular spaces without mitotic figures or tissue invasion. Magnetic resonance imaging identified subcutaneous nodules with hyperintense signals on T1 and T2 imaging, consistent with IPEH. The patient was managed conservatively after unsuccessful sclerotherapy and opted for periodic monitoring.
Keywords
Atypical
Histopathology
Intravascular papillary endothelial hyperplasia
Magnetic resonance imaging
Masson’s tumour
Radiology
INTRODUCTION
Masson’s tumor or intravascular papillary endothelial hyperplasia (IPEH) is a rare, benign intravascular lesion, constituting 2–4% of overall skin vascular tumors. It is deemed to be a reactionary proliferation of endothelial cells and is frequently associated with organized thrombus in the presence of angiogenic factors, presenting as a bluish to red, firm or soft, nonpulsatile nodule that is non-adherent to deeper planes.1 IPEH is known to occur in all age groups, with a female preponderance, and shows a distinct preference to sites such as head, neck and extremities, and usually occurs as a solitary lesion.2 However, lately, a few atypical sites such as the parotid gland, kidneys, eyelid, and sinonasal cavity have been reported.3 IPEH represents a diagnostic dilemma as it bears a close resemblance to angiosarcoma. Here, we report a rare atypical case of IPEH, presenting with multiple nodules in widely distributed areas, along with comprehensive clinical-histopathological and radiological correlation. This is the first case of distant multi-site primary IPEH reported in Indian literature to the best of our knowledge.
CASE REPORT
An otherwise healthy 37-year-old male patient presented with multiple asymptomatic nodules on the wrist and lateral aspect of the left forearm along with two solitary nodules, one on the left thigh and another on the flexural aspect of the right forearm, which had persisted for 4 years. There was no history of prior trauma at these sites, or of similar lesions in the past or in his family. He took non-steroidal anti-inflammatory drugs, indigenous medications, and topical corticosteroids from local practitioners but the lesions persisted. Physical examination revealed superficial, blue to reddish, soft, monomorphic, mobile nodules, ranging from 1 to 2 cm diameter in size [Figure 1a-d]. Dermoscopic evaluation of the lesion on the left forearm revealed multiple bright red to purple multi-coloured vascular areas in the center (rainbow pattern) [Figure 1e]. Clinical differential diagnoses of Hemangioma, Kaposi Sarcoma, Superficial Glomuvenous malformation, Dabska tumour, Angiosarcoma, and IPEH were kept. Biopsy from a nodule revealed a lesion composed of deep-seated vascular dilatation that was shelled out from the rest of the dermis, with its walls resembling the structure of a vein. Within the lumen was reticulate proliferation of similar, although smaller vessels some of which had papillomatous outlines with endothelial hyperplasia. There was absence of mitotic figures, necrosis, nuclear pleomorphism, and infiltration into adjacent tissues [Figure 2a and b]. Therefore, the diagnosis of Masson’s tumor (primary type) was confirmed.
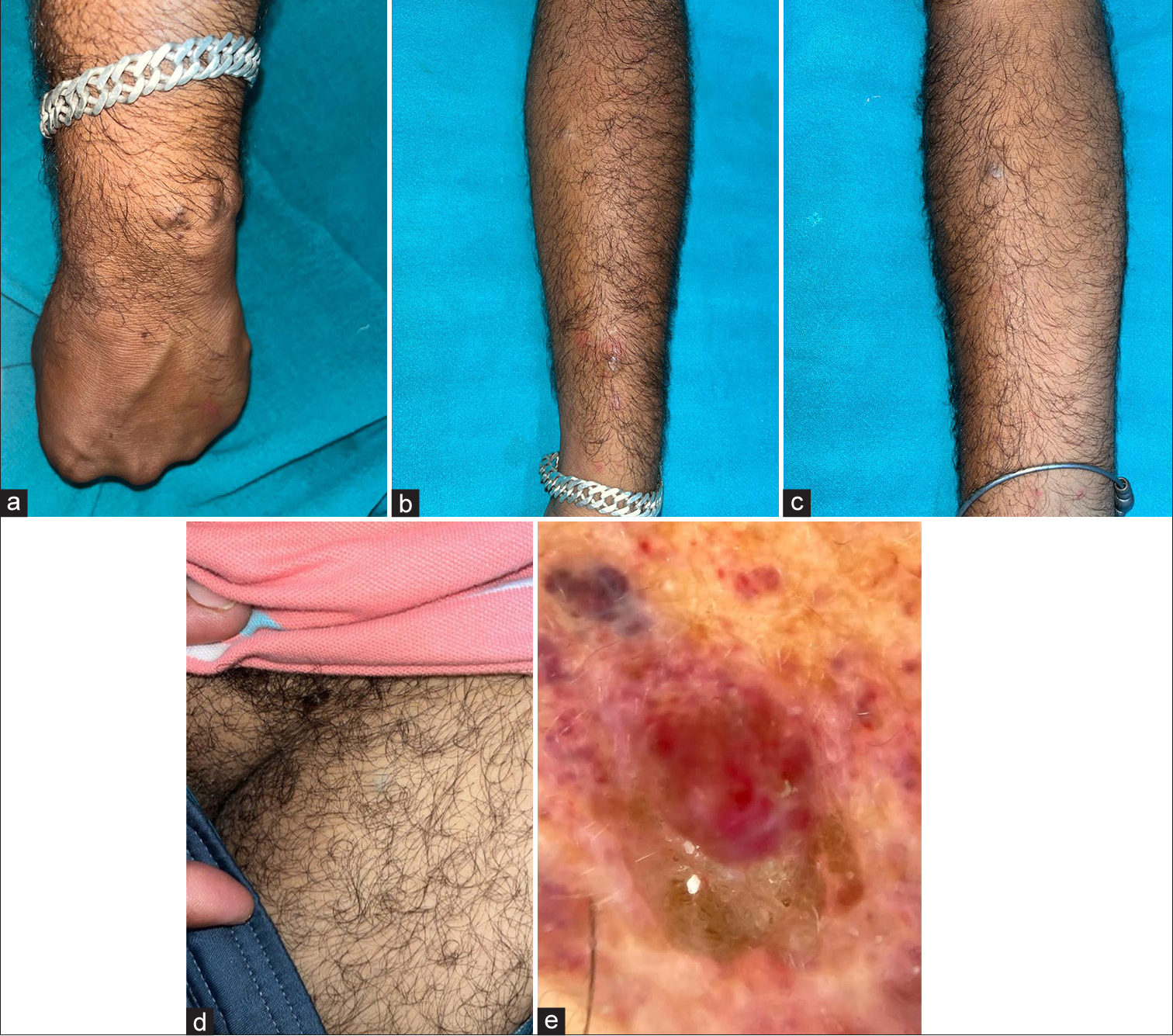
- (a) Nodule on the posteromedial aspect of the left wrist. (b) Nodules on the lateral aspect of the left forearm. (c) Showing nodule on the anterolateral aspect of the right forearm. (d) Nodule on the medial aspect of the left thigh. (e) Dermoscopic image showing rainbow pattern in the lesion.
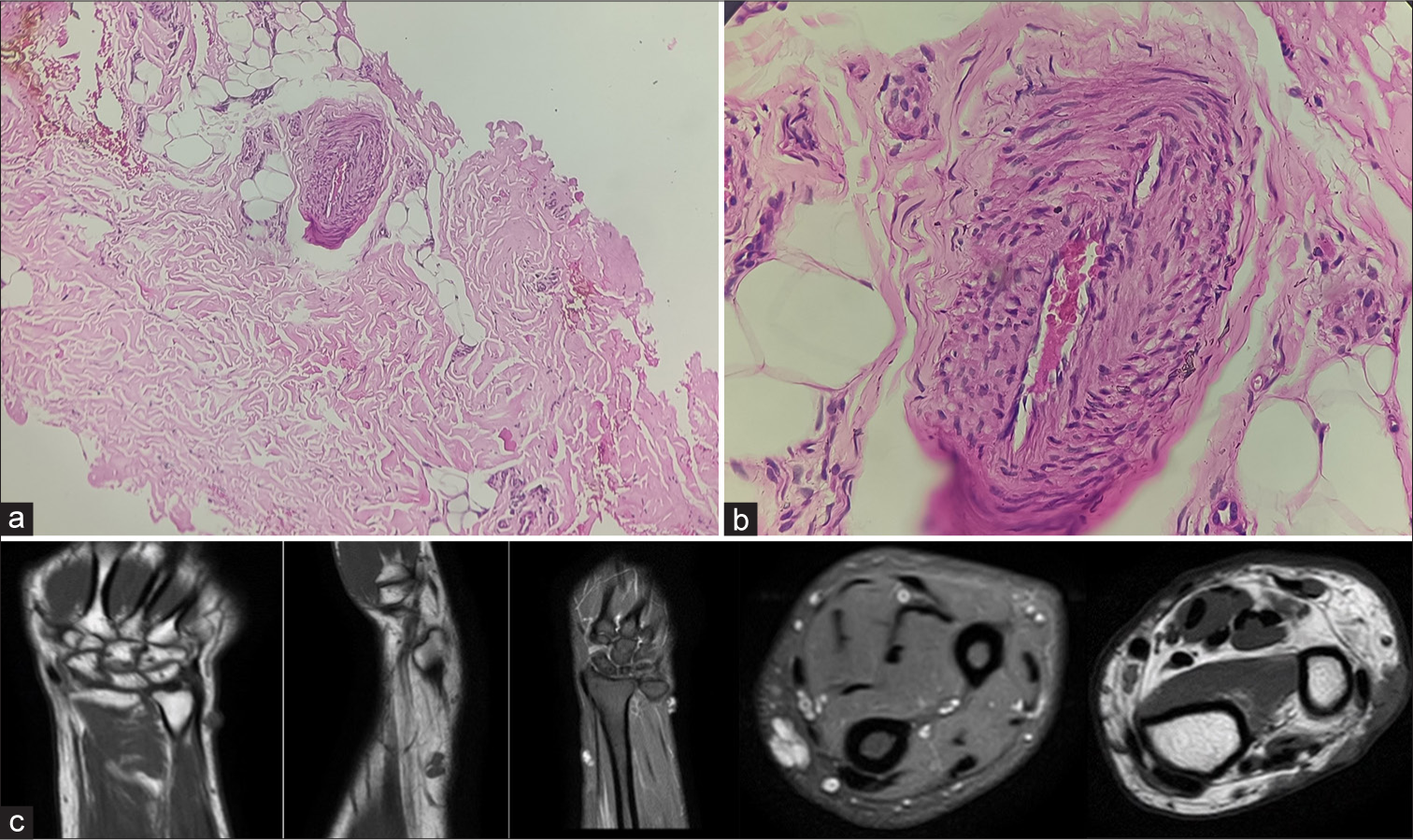
- (a) Microscopic section shows a ×100 view of a proliferative vascular lesion shelled out from rest of the dermis (hematoxylin and eosin stain) and (b) microscopic section shows a ×400 view of lumen of the proliferative vessel having papillomatous outline with endothelial hyperplasia (hematoxylin and eosin stain). (c) Multiple magnetic resonance imaging sections demonstrating heterogenous insinuating nodules in the subcutaneous plane along the extensor compartment of distal forearm appearing hyperintense on T1- and T2-weighted images.
Magnetic resonance imaging (MRI) of the right forearm was suggestive of an ill-defined, heterogeneous, insinuating nodules in the subcutaneous plane along the extensor compartment of the distal forearm measuring 15 × 7 mm in size with another similar signal intensity lesion in the mid-forearm along the radial aspect. It appeared hyperintense on T1- and T2-weighted images. Few prominent venous channels were observed in the distal forearm along the flexor aspect (ulnar side) in subcutaneous fat [Figure 2c]. The patient underwent a trial session of intralesional sclerotherapy using sodium tetradecyl sulfate for a solitary lesion. However, no reduction in lesion size was observed. Subsequently, surgical excision was recommended, but the patient elected to pursue conservative management. This included periodic follow-up every 6 months, avoidance of trauma, and monitoring for changes such as lesion enlargement, local infections, ulceration, or bleeding. The patient was followed for a duration of 1.5 years, during which the lesion remained asymptomatic and stable in size. Thereafter, the patient was lost to follow-up.
DISCUSSION
IPEH was first described by Pierre Masson in 1923 as an intravascular vegetating hemangio-endothelioma, but the current nomenclature was proposed by Clearkin and Enzinger in 1976.4 It is a benign vascular hyperplasia of a single layer of endothelial cells covering a bundle of hyalinized papillary growth. Over the past decades, it has been characterized in various anatomical locations within the soft tissues of the body.5 It can manifest in three distinct types: primary, secondary, and extravascular. The primary type typically presents as a single asymptomatic, firm, bluish nodule predominantly affecting the deep dermis and subcutaneous tissues of the head, neck, fingers, and trunk. Oral mucosa, lip, thyroid, maxillary sinus, parotid, lung, superior vena cava, adrenal gland, renal vein, forearm, foot, and the intracranial sites have also been reported.6 The secondary type is correlated with the primary tumor and is frequently associated with deep-seated hemangiomas. The extravascular type, which is the least common, is associated with hematomas.
The precise etiology of IPEH remains unclear; however, it is hypothesized that its formation may result from repetitive microtrauma. Pathogenesis of IPEH involves atypical thrombus formation and β-fibroblast growth factor. Immunohistochemical analysis shows positive expression for Wilms tumor 1 (WT1), CD31, CD34, α-smooth muscle actin, and Factor VIII while typically negative for glucose transporter 1 (GLUT 1). The CD105 marker, exclusive for true primary vascular tumors, helps differentiate IPEH from other vascular lesions, such as angiosarcoma.7 Malignant soft-tissue neoplasms are important differential diagnosis of IPEH [Table 1]. Diagnosing IPEH at atypical sites can be difficult due to its intermittent symptoms and, at times, recurrent bleeding.8 Imaging studies, such as ultrasonography, may distinguish IPEH from other soft-tissue tumours by detecting vascular component of the lesion. MRI may reveal low-signal intensity on T2-weighted sequences, indicating the presence of thrombotic or hemorrhagic material with low-signal internal septa. Depending on size, location, symptoms, and rate of growth, surgical excision remains the hallmark of treatment options.7,9 As it is a benign lesion, treatment of choice is complete surgical resection without a need for wide margins due to the low risk of recurrence; however, incomplete excision may influence recurrence rates, which in long-term are reported to up to 15%. Pre-operative radiological delineation of lesion boundaries is critical for minimizing morbidity and ensuring complete removal, particularly in anatomically complex regions. Aggressive forms of IPEH, which have higher recurrence rates can be subjected to chemotherapy and radiation on recurrence.10 Other treatment options in the literature include vascular lasers and decompression therapy.11 However, the evidence for these is limited. The small asymptomatic primary IPEH lesions can remain stable for years, may progress, and complicate with recurrent bleeding, ulcerations, pain, and compression symptoms of adjacent neurovascular bundle, or rarely regress. Secondary IPEH also has a higher rate of post-surgical recurrence if the underlying condition is not addressed.
| Diagnosis | Cytological atypia | Necrotic foci | Papillary structures | Endovascular location | Stratification of epithelial cells |
|---|---|---|---|---|---|
| Angiosarcoma | Common | Common | Rare | Rare | Common |
| Kaposi sarcoma | Common | Uncommon | No | Rare | Rare |
| Cavernous/capillary hemangioma | Rare | No | No | No | Rare |
| IPEH | Rare | No | Common | Common | Rare |
IPEH: Intravascular papillary endothelial hyperplasia
CONCLUSION
Histopathological and radiological findings taken in tandem with clinical features of the disease can help diagnose, differentiate, and treat IPEH while avoiding unnecessary aggressive treatments.
Authors’ contributions:
All the authors contributed equally to the manuscript writing.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Multiple nodules on the unilateral upper limb. JAAD Case Rep. 2022;29:59-61.
- [CrossRef] [PubMed] [Google Scholar]
- Intravascular papillary endothelial hyperplasia in the oral mucosa and jawbones: A collaborative study of 20 cases and a systematic review. J Oral Pathol Med. 2021;50:103-13.
- [CrossRef] [PubMed] [Google Scholar]
- Intravascular papillary endothelial hyperplasia (Masson's Tumor): Diagnosis the plastic surgeon should be aware of. Plast Reconstr Surg Glob Open. 2017;5:e1122.
- [CrossRef] [PubMed] [Google Scholar]
- Intravascular papillary endothelial hyperplasia. Arch Pathol Lab Med. 1976;100(8):441-4.
- [Google Scholar]
- Masson's tumor of the hand: An uncommon histopathological entity. Case Rep Pathol. 2020;2020:4348629.
- [CrossRef] [PubMed] [Google Scholar]
- Intravascular papillary endothelial hyperplasia (Masson Tumor) of the right thumb: A case report and literature review. Plast Reconstr Surg Glob Open. 2023;11:e5224.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary endothelial hyperplasia (Masson Tumor) of the hand. Surgical and pathological consideration from seven cases using new vascular markers. Pathol Oncol Res. 2020;26:2083-90.
- [CrossRef] [PubMed] [Google Scholar]
- Masson's tumor as a rare source of obscure small bowel bleeding: 950. Am J Gastroenterol. 2013;108:S284.
- [CrossRef] [Google Scholar]
- Intravascular papillary endothelial hyperplasia. A clinicopathologic study of 91 cases. Am J Dermatopathol. 1983;5:539-46.
- [CrossRef] [PubMed] [Google Scholar]
- Intravascular papillary endothelial hyperplasia (Masson's Tumor) involving the knee synovium. J Orthop Case Rep. 2018;8:23-5.
- [Google Scholar]
- Recurrent intravascular papillary endothelial hyperplasia of the right middle finger treated with radiation therapy. J Bone Joint Surg Br. 2008;90B:95-7.
- [CrossRef] [PubMed] [Google Scholar]







