Translate this page into:
A Comparative Study on Therapeutic Efficacy of Autologous Platelet-rich Plasma, Autologous Platelet-rich Fibrin Matrix, Recombinant Human Epidermal Growth Factor, and Collagen Particles in Nonhealing Leg Ulcers
Address for correspondence: Dr. Shikha R. Shah, Department of Dermatology, Room Number 139, Wing Number 3, OPD Building, BJ Medical College (BJMC), Civil Hospital, Ahmedabad, Gujarat, India. E-mail: srs4894@gmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Nonhealing leg ulcers are challenging to manage and cause significant patient morbidity. To promote healing, newer techniques focus on delivering/enhancing dermal matrix components.
Aim:
The aim of this study was to compare the therapeutic efficacy of autologous platelet-rich plasma (PRP), autologous platelet-rich fibrin matrix (PRFM), recombinant human epidermal growth factor (rhEGF), and collagen particles in treating nonhealing leg ulcers.
Materials and Methods:
Open, randomized prospective study was conducted in a single tertiary center over 2 years where after fulfilling the criteria, randomization was done into four groups. Group A: Autologous PRP (double spin, manual method, weekly); Group B: Autologous PRFM (weekly); Group C: rhEGF (daily application); and Group D: Collagen particles (weekly) along with cleansing, debris removal, and wound dressing. Treatment endpoints were complete healing/6 months of treatment, whichever was earlier. Follow-up was done two weekly by clinical assessment, photographs, and measurement of the ulcer area. Epi info 7 software was used for statistical analysis.
Results:
A total of 48 patients completed the study, 12 in each group, with mean age: 42.37 ± 4.56 years and male-to-female ratio 2.6:1. Underlying etiology was varicosities (43.75%), traumatic (25%), diabetes (22.91%), and leprosy (8.34%). At baseline, all groups were comparable in terms of patient and ulcer characteristics. Complete healing was seen in 79.17% at the end of 12 weeks: 91.67% of patients from Groups A and B each, and 66.67% from Groups C and D each. The mean time to complete healing was 6.9 ± 2.5 weeks, the least in Group B (4.73 ± 2.3 weeks). Differences between excellent (≥75%) ulcer healing across all groups were statistically significant at the end of 8 weeks where Group B showed maximum improvement. No major adverse events were seen.
Conclusion:
PRFM resulted in relatively faster ulcer healing compared with other modalities.
Keywords
Collagen particles
epidermal growth factor
nonhealing ulcer
platelet-rich fibrin matrix
platelet-rich plasma
wound healing
Management of nonhealing leg ulcers poses a therapeutic challenge. This prospective study shows superior efficacy of platelet-rich fibrin matrix, a cost-effective and feasible modality, over platelet-rich plasma, recombinant epidermal growth factor, and collagen particles, with a mean duration of complete healing in 4–5 weeks with excellent patient acceptability

INTRODUCTION
Nonhealing leg ulcer is defined as a chronic defect in the skin below the level of knee persisting for more than 6 weeks and shows no tendency to heal after 3 or more months.[1] The incidence of ulceration is rising due to increasing risk factors for atherosclerotic occlusion such as smoking, obesity, and diabetes.[1] Prevalence of nonhealing ulcers in India is found to be 4.5/1000 population.[2] Regardless of the underlying etiology, nonhealing ulcers tend to have chronic pain, discharge, sleep impairment, and subsequent adverse repercussions in quality of life; posing many therapeutic challenges. Principles of therapy are addressal to the underlying cause, symptom management, interventions to improve circulation, and promotion of wound healing.
Autologous platelet-rich plasma (PRP) provides anti-inflammatory action on the wound bed as well as contents of the alpha granules (platelet-derived growth factors and vascular endothelial growth factors) released locally to stimulate the initiation of healing.[3] Autologous platelet-rich fibrin matrix (PRFM) provides growth factors, especially transforming growth factor beta (TGF)-beta which is profibrotic, increases biomechanical strength, and helps in epithelial resurfacing and differentiation.[4] Epidermal growth factor (EGF) is quintessential for wound healing. By acting on epithelial cells and fibroblasts, recombinant human epidermal growth factors (rhEGF) promote faster re-epithelization.[5] Collagen dressings act as a scaffold as well as spare the healthy collagen by acting as a decoy for the wound matrix metalloproteinases, thereby accelerating wound healing.[6]
The aim of this study was to compare the therapeutic efficacy of PRP, PRFM, rhEGF, and collagen particles: four relatively newer modalities in the management of nonhealing ulcers.
SUBJECTS AND METHODS
An open-labeled, randomized prospective interventional study was conducted in the Department of Dermatology in a single tertiary care center after informed patient consent and approval by Institutional Ethical Committee from July 2018 to August 2020. The inclusion criteria of the study were patients aged 18–65 years and either sex having nonhealing ulcers on the lower extremity of any etiology, defined as ulcer ≥1 year duration and/or showing no/inadequate signs of healing after 12 weeks of appropriate treatment. The exclusion criteria of the study were pregnant and lactating females, bleeding disorders, and/or oral anticoagulant therapy, uncontrolled diabetes, proven malignancy, actively infected ulcers, ulcers >10 cm2 area, ulcers with exposed bone with no underlying granulation tissue, HIV/AIDS and those with unrealistic expectations and unwilling to give consent for treatment or photography. The detailed case history was elicited as per a predesigned proforma. General, systemic and local examination was performed noting the attributes of the ulcer, surrounding skin, and regional lymph nodes. All patients were subjected to the following baseline investigations: complete blood count, liver and renal function tests, coagulation profile PT/INR, APTT, random blood sugar, HbA1c, viral markers––HIV, HBsAg, HCV, and ultrasonography with Doppler of limbs. Ulcers cleansing, slough debridement, and treatment of the underlying condition(s) were done. Fifty consecutive patients fulfilling the study criteria were randomly allocated into one of the four treatment arms [Figure 1]:
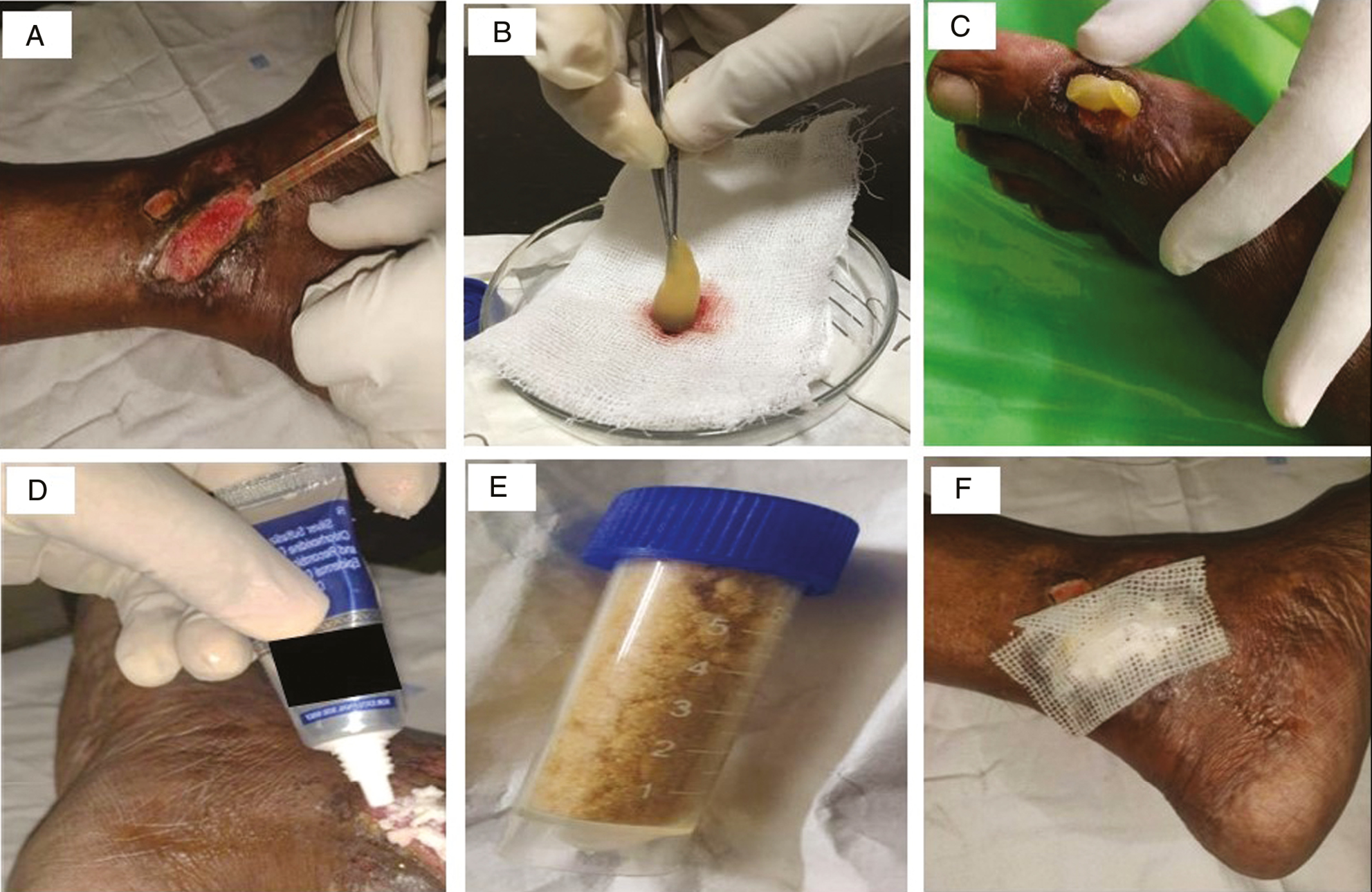
- Therapeutic modalities employed for nonhealing ulcer in this study are depicted. (A) Injection of autologous PRP into the base and sides of the ulcer in Group A. (B and C) Separation of PRFM from adherent blood clot and placement over the cleansed wound bed, in Group B. (D) Application of rhEGF cream to the wound in Group C. (E) Collagen particles in Group D to be sprinkled adequately over the wound. (F) Paraffin-impregnated non-adherent gauze dressing applied after the procedure
Group A: Autologous PRP
Group B: Autologous PRFM
Group C: rhEGF
Group D: Collagen particles
Group A: Autologous platelet-rich plasma
PRP was done manually by the double-spin technique. Under aseptic conditions, 20 mL of whole blood was withdrawn and collected in the centrifuge tube prefilled with Anticoagulant Citrate Dextrose (1.5 mL each) and rotated at 2000 rpm for 15 min (soft spin). The buffy coat along with plasma was transferred into sterile tubes without anticoagulant with the help of a pipette and rotated at 3000 rpm for 10 min (hard spin). The upper two-third of the supernatant was discarded and the remaining, that is, PRP was filled in insulin syringes. After disinfecting the wound area using betadine solution and excising the necrotic slough, PRP was injected into the base of the ulcer and the surrounding skin. Sessions were repeated weekly for a maximum of six sessions.
Group B: Autologous platelet-rich fibrin matrix
Under proper asepsis, 10 mL of whole blood was withdrawn, collected in a centrifuge tube without anticoagulant, and subjected to a single spin at 3000 rpm for 10 min (hard spin only). Three layers were obtained following this: upper straw-colored platelet-poor plasma (PPP), red-colored lower fraction containing red blood cells (RBCs), and the middle fraction containing the PRFM. The upper straw-colored layer (PPP) was discarded. PRFM was separated from red corpuscles at the base using sterile forceps and scissors, preserving a small RBC layer measuring around one mm in length, which was transferred onto a sterile gauze. The middle membrane so obtained was compressed between two gauze pieces gently and applied on a healthy wound followed by the application of a secondary non-absorbable dressing. Sessions were repeated weekly for a maximum of six sessions.
Group C: Recombinant human epidermal growth factor
Combination of 10 µg recombinant human epidermal growth factor gel (rhEGF) with 1% w/w silver sulfadiazine and 0.2% w/w chlorhexidine gluconate was applied to the clean wound in a thin layer followed by sterile dressing with paraffin impregnated gauze and a secondary dressing. Treatment frequency was once a day application.
Group D: Collagen particles
After wound cleansing, commercially available sterile collagen particles with 2% w/w mupirocin and 1% w/w metronidazole were sprinkled sufficiently to cover the wound surface. Paste or solution with normal saline was used to ensure particle delivery in deeper or undermined wounds, covered by the absorbent dressing. This treatment was repeated weekly.
Treatment endpoints were complete healing of the wound or six months of treatment, whichever was earlier. Follow-up was done every 2 weeks with clinical photography objective assessment of ulcer size, by measuring the greatest length and breadth via thread and scale (clock-face method). Physicians Global Assessment (PGA) scoring was done where a decrease in ulcer size by <25% was defined as no/poor improvement, 25%–50% as some improvement, 50%–75% as good improvement, and ≥75% as an excellent improvement. Ancillary subjective assessment was done using visual analog scale (VAS) for pain and Dermatology Life Quality Index (DLQI).
Statistical analysis was done using Epi info 7 software. Frequency and percentage were calculated for qualitative data and mean and standard deviation was calculated for quantitative data. In qualitative data, the chi-square test was used to calculate statistically significant differences among various groups, whereas t test or ANOVA was used for quantitative data. A value of P < 0.05 was considered statistically significant.
RESULTS
From a total of 52 patients fulfilling the study criteria, 48 completed the study: 12 patients in each of the four groups and were included in the final analysis, whereas four were lost to follow-up (one in Groups A and B each due to lack of feasibility to return for the procedure, and two from Group C due to lack of response). The mean age of patients was 42.37 ± 4.56 years with a range of 32–56 years. The male-to-female ratio was 2.6:1 with 72.9% (n = 35) males and 27.1% (n = 13) females. The most common occupation was manual labor in 35.4% (n = 17) patients and 52.1% (n = 25) belonged to lower socio-economic status. The most common presenting complaints were pain and discharge from the ulcer in all groups. Underlying comorbidities eventuating into ulcers were varicosities in 43.75% (n = 21), followed by traumatic in 25% (n = 12), diabetes in 22.91% (n = 11) and leprosy in 8.34% (n = 4). Addiction in the form of alcohol intake was seen in 31.3% (n = 15), smoking in 27.1% (n = 13), and both in 31.3% (n = 15) patients. The majority of patients 52% (n = 25) had ulcers for the past 8 to 10 months duration, 25% (n = 12) patients had an ulcer for more than 10 months, and 23% (n = 11) patients had an ulcer for less than 8 months. At baseline, all groups were comparable in terms of baseline characteristics as well as ulcer size and duration (P > 0.05).
Table 1 shows the decline in the area of the ulcer from baseline at the end of 12 weeks. Figure 2 shows the differences between groups in terms of ulcer healing at 8 and 12 weeks.
| Mean area of the ulcer at baseline | Mean area of the ulcer at 12 weeks | Mean percentage decrease in ulcer size | |
|---|---|---|---|
| Group A | 6.75 ± 0.97 cm2 | 0.26 ± 0.86 cm2 | 96.15% |
| Group B | 5.89 ± 1.72 cm2 | 0.16 ± 0.58 cm2 | 97.28% |
| Group C | 6.63 ± 0.97 cm2 | 0.45 ± 0.82 cm2 | 93.21% |
| Group D | 5.81 ± 0.80 cm2 | 0.56 ± 0.80 cm2 | 90.36% |
Maximum improvement was seen in Group B (PRFM modality)
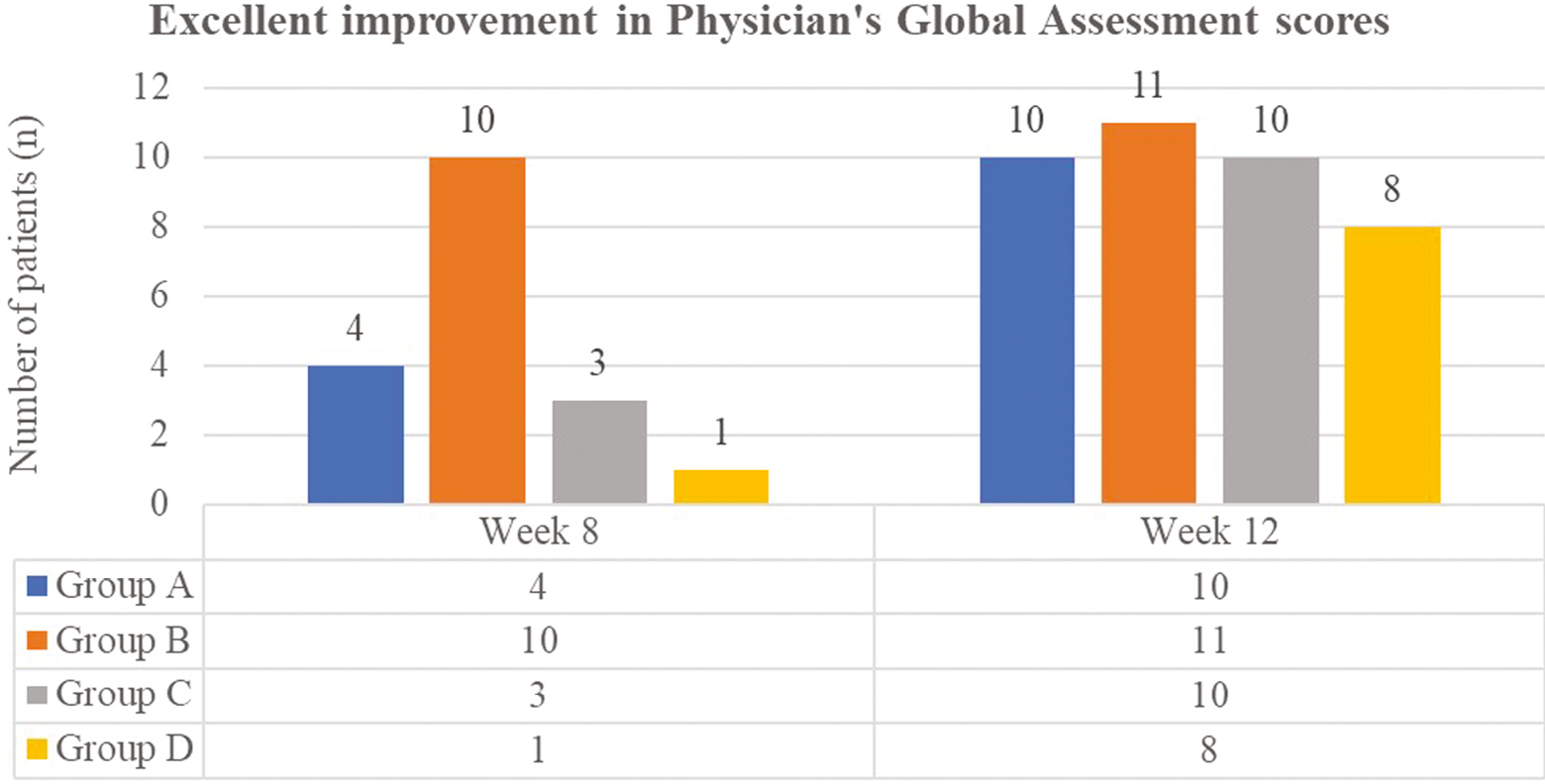
- Excellent improvement in Physicians’ Global Assessment scores. At the end of 8 weeks, excellent ulcer improvement* was seen in majority of the Group B patients (PRFM arm). This difference between groups was statistically highly significant (P = 0.0001). At the end of 12 weeks, difference between excellent ulcer healing between groups was statistically insignificant (P = 0.05)
- (*≥75% reduction in the size of the ulcer from baseline was defined as excellent improvement)
Early endpoint (8 weeks)
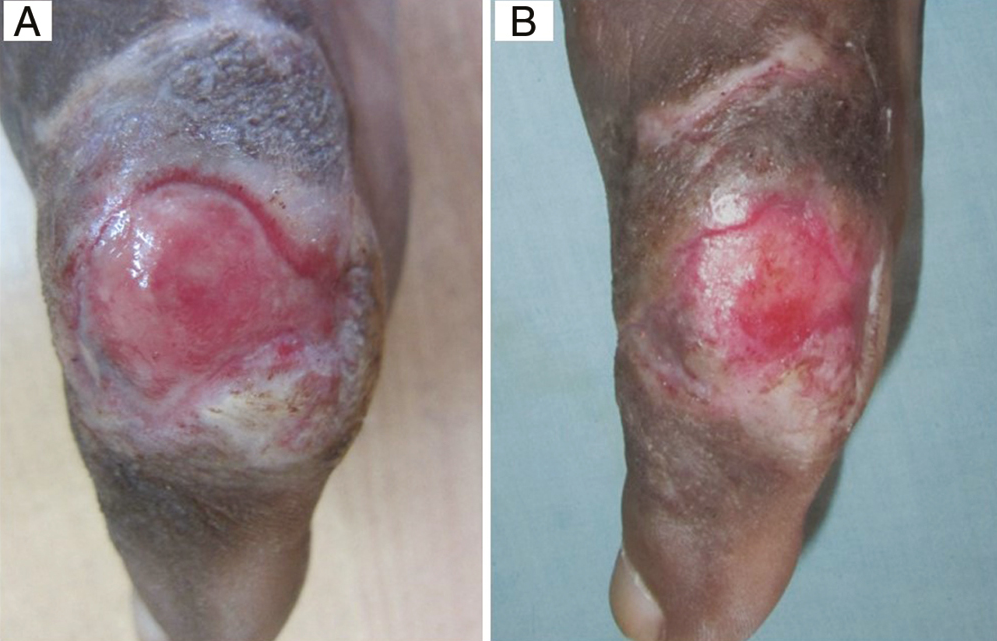
On applying the Physician Global Assessment score, excellent improvement (>75%) was achieved in a total 18 out of 48 patients, that is, 37.5% of patients at the end of 8 weeks, with improvement in maximum patients of Group B, 83.33%, that is, 10 out of 12 patients, followed by Group A (33.33%, n = 4), Group C (25%, n = 3), and Group D (8.33%, n = 1) [Figures 3,4,5–6]. The difference in ulcer healing at 8 weeks was statistically highly significant (P = 0.0001).
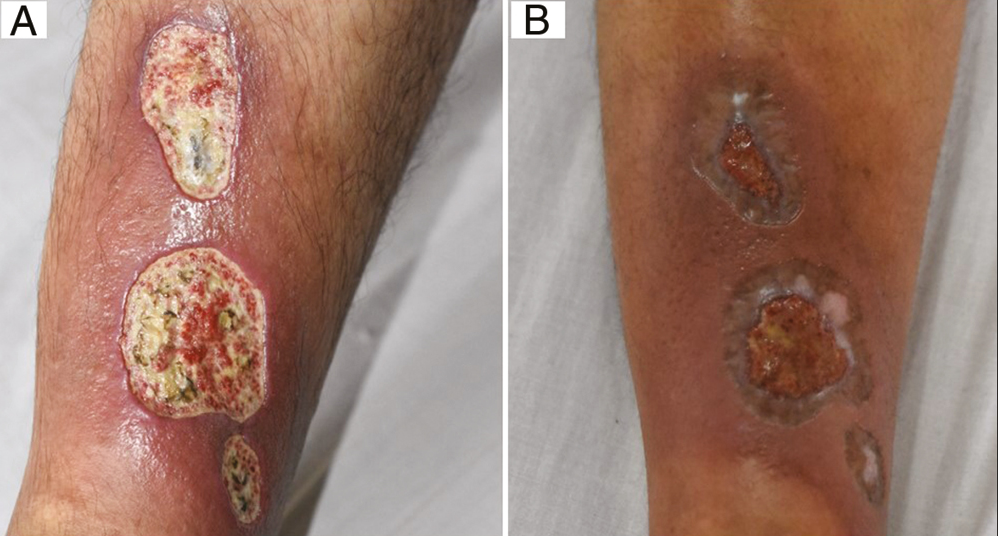
- (A) Baseline: Posttraumatic ulcer in a 34-year-old man. (B) Week 8: After six sessions of autologous platelet-rich plasma therapy (Group A)
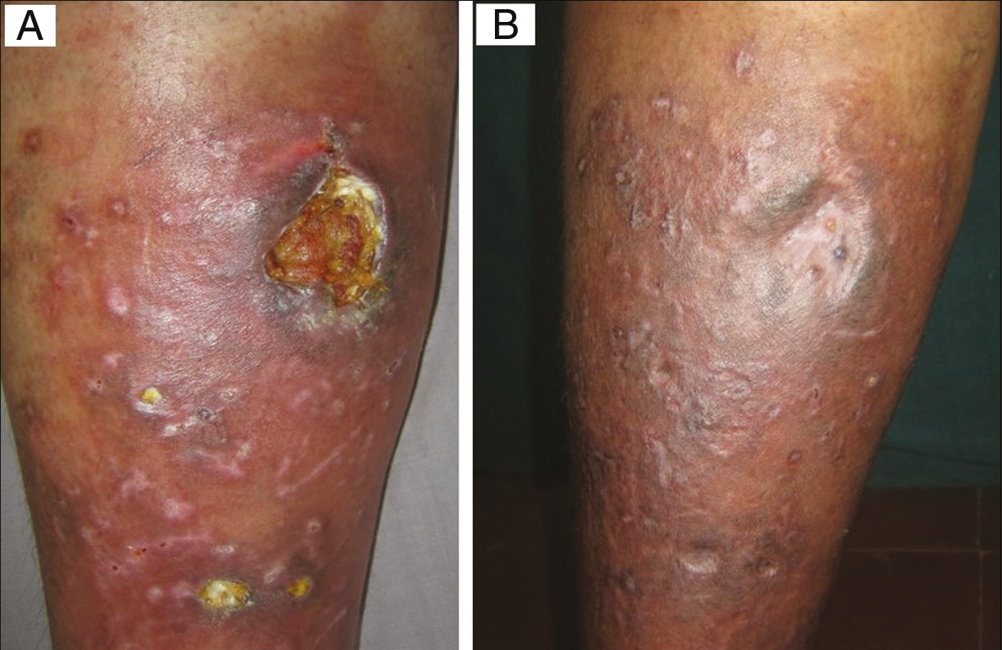
- (A) Baseline: Varicose ulcer in a 54-year-old man. (B) Week 8: After four sessions of autologous platelet-rich fibrin therapy (Group B)
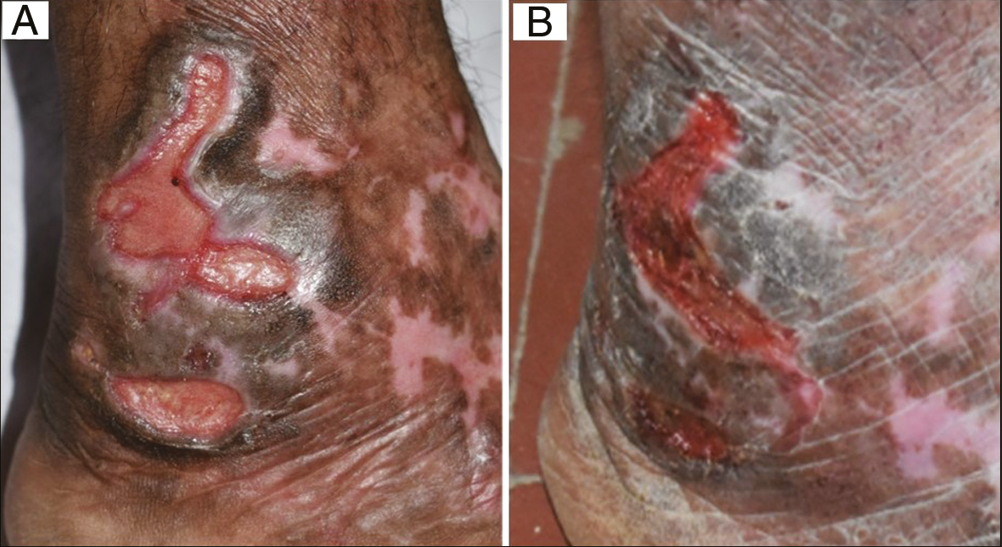
- (A) Baseline: Trophic ulcers in a 56-year-old diabetic woman. (B) Week 8: After recombinant human epidermal growth factor application (Group C)
- (A) Baseline: Trophic ulcers in a 30-year-old man with leprosy. (B) Week 8: After collagen particle dressing (Group D)
Late endpoint (12 weeks)
At the end of 12 weeks, excellent improvement in PGA was seen in total 41 out of 48, that is, 85.41% of patients, with maximum patients improving in Group B (91.67%, n = 11) followed by Groups A and C (83.33%, n = 10 each), and Group D (66.67%, n = 8). The difference in ulcer healing at 12 weeks was not statistically significant (P = 0.05).
Complete healing
Complete healing was seen in 79.17% of patients, that is, in 38 out of total of 48 patients at the end of 12 weeks, which comprised 91.67% of patients (n = 11) from Groups A and B each, followed by 66.67% (n = 8) from Groups C and D each. The rest 10 patients showed partial healing.
Overall, the mean time to complete the healing of the ulcer was 6.9 ± 2.5 weeks. Mean time to complete healing was the least, that is, 4.73 ± 2.3 weeks in Group B followed by 7.46 ± 2.43 weeks in Group A weeks, 7.63 ± 1.8 weeks in Group C 7.63 ± 2.06 weeks in Group D. The differences in healing time were not statistically significant.
From the 38 patients showing complete healing, a healing time of <4 weeks was seen in 13.16% (n = 5) patients, comprising n = 4 from Group B and n = 1 from Group A. Healing time of 4–8 weeks in 42.11% (n = 16 patients), comprising n = 6 patients from Group B, followed by n = 4 from Group A and n = 3 from Group C and Group D each. Healing time of 8–12 weeks in 44.73% (n = 17) patients, comprising n = 6 patients from Group A, followed by n = 5 from Group C and Group D each, and n = 1 from Group B.
There was a significant decline in the VAS scores and improvement in DLQI in all groups at week 12 compared to baseline, with no statistically significant differences between the treatment modalities. All treatment modalities were tolerated by most patients with no adverse events in the study, except for local irritation complained by two patients in Group C and minor swelling/bruising during blood sampling in Groups A and B.
DISCUSSION
In the Indian context, nonhealing leg ulcers may stem from systemic conditions such as diabetes, atherosclerosis, leprosy, venous ulcers, pressure ulcers, vasculitis, and inappropriate management of acute trauma.[2] Despite adequate preparation of wound bed, infection control, and a moist environment, chronic leg wounds may fail to respond, necessitating the use of techniques that deliver and enhance dermal matrix components, that is, growth factors, fibrin preparations, and/or scaffold proteins.[7] In our study, the majority of patients achieved complete ulcer healing in all four modalities with excellent patient acceptability, but PRFM showed slightly better efficacy along with faster wound healing [Figure 7]. Comparison between studies in the literature is difficult, due to differences in the underlying etiologies, procedural and protocol variations, and lack of similar head-on comparative studies between the four modalities in this cohort.
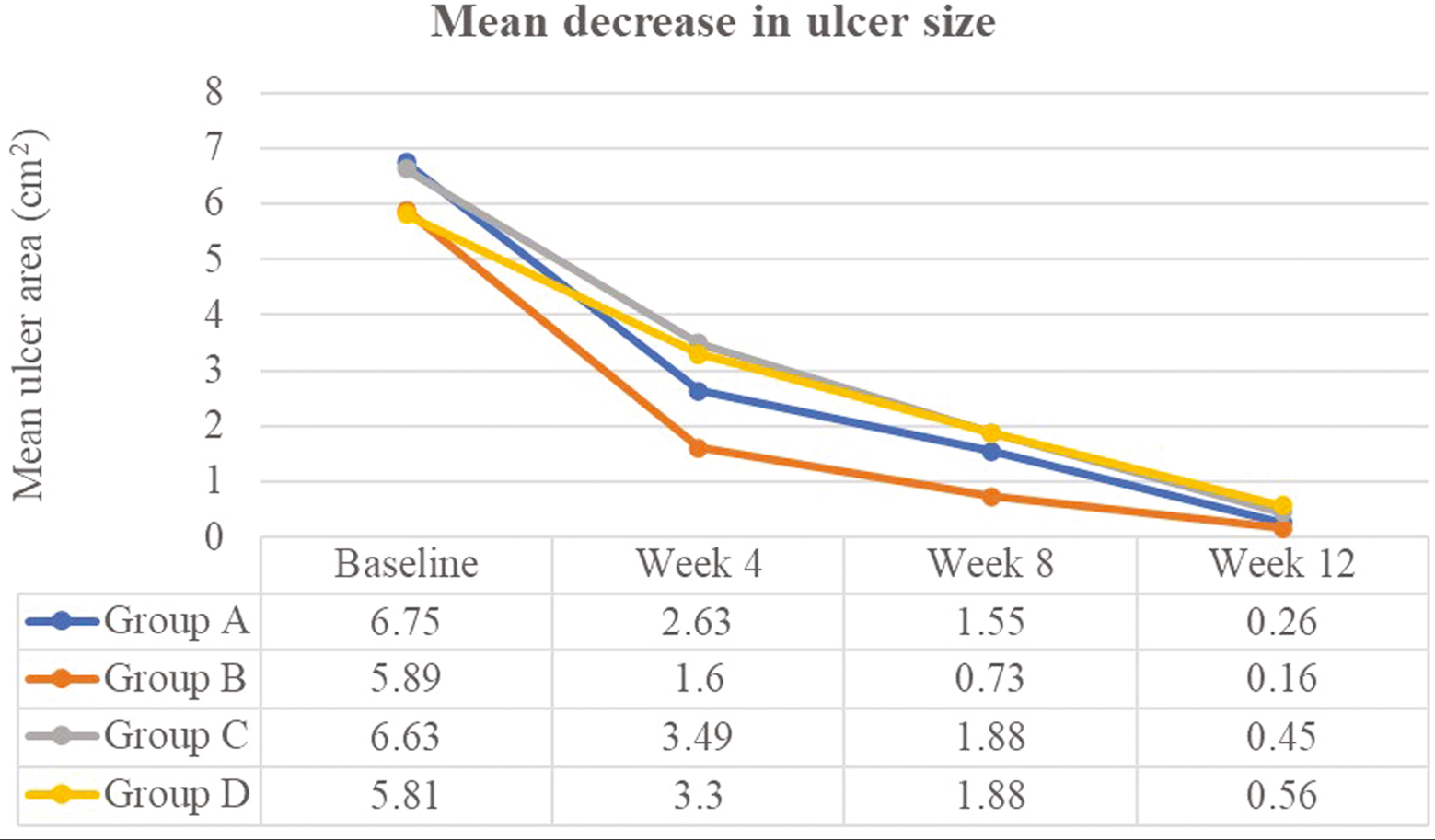
- Mean decrease in ulcer size. Comparison of time needed for decline in the mean ulcer size at 4, 8, and 12 weeks of therapy across all groups is depicted. Faster healing times were seen with Group B (PRFM arm)
In a landmark study by Margolis et al.[8] which included 26,599 patients, it was found that ulcers treated with “platelet releasate” tended to heal faster than patients who were treated without them. PRFM due to its fibrinous matrix acts as a novel natural carrier to deliver three-fold higher growth factor concentrates to the wound bed with a controlled release, versus PRP despite the latter having higher platelet concentrates.[910] This theoretically translates into superior efficacy of PRFM over PRP. In our study, both PRFM and PRP showed ulcer resolution in 91.67% of patients albeit faster with the PRFM group, versus a recent randomized controlled trial by Pravin et al.,[11] where 73.3% and 53.3% of patients achieved ulcer clearance with PRFM and PRP, respectively. Mean time to complete healing in our study was 4.73 weeks (corresponding to approximately four to five sittings), which is comparable with Sarvajnamurthy et al.[12] and Dorjay et al.,[13] where complete healing took approximately 5 weeks (corresponding to 5 sittings). Apart from providing a plethora of growth factors, PRFM behaves like a biologically active tissue, akin to tissue graft for cutaneous wounds.[14] It contains leucocytes which help combat and prevent secondary infections, enabling its use across leg ulcers of multiple causes.
EGF family comprises four proteins: EGF, TGF-alpha, heparin-binding EGF, and amphiregulin.[15] Acting as a mitogen, EGF stimulates the proliferation and migration of keratinocytes, granulation tissue formation, and fibroblast motility.[5] Recombinant human EGF has the best evidence for use in diabetic ulcers. Recent meta-analysis involving 530 patients showed that intralesional injection and/or topical application of rhEGF in diabetic foot ulcers had better rates of healing than conventional modalities (combined odd’s ratio of 4.005, P < 0.001).[16] The indications have now expanded to nonhealing ulcers of other etiologies. Sachez et al.[17] studied rhEFG in chronic venous ulcers and found that 100% of ulcers re-epithelialized; 71% achieved wound closure in 8 weeks or less, and the remaining within 9 and 12 weeks. This was comparable with our subset of five patients in Group C with venous ulcers of which four, that is, 80% of patients healed completely in approximately 8–10 weeks. There is a paucity of studies that compare the efficacy of this modality to platelet-derived modalities/ collagen dressings. A recent study on microbiome colonization showed that patients treated with EGF had no infections during the follow-up period, and there was a significant difference versus patients treated with PRP who had Staphylococcus aureus and Pseudomonas aeruginosa colonization (P = 0.0078).[18] However, no patient in this study had infections either in PRP or rhEGF arm.
Collagen is fundamental to wound healing. Chalimidi et al.[6] found a significant decrease in wound size by using collagen particles versus conventional dressings. Collagen particle dressings belong to protease-modulating matrix (PMM) treatments that improve healing by physically removing proteases from the wound fluid. Evidence regarding the superiority of PMM over non-PMM concept dressings is unclear in literature as per a recent review of 784 patients.[19] Qureshi et al.[20] conducted a study on 102 patients with nonhealing ulcer and found PRP to be safer and more effective with faster healing rates and time compared with collagen dressing (P < 0.05); a similar finding was seen in our study. Durvasula et al.[21] did a comparative study on 30 patients with trophic ulcers secondary to leprosy>diabetes>venous insufficiency, comparing the efficacy of collagen particles impregnated with antibiotics (similar to our cohort) with PRFM. Complete healing was seen in 80% and 53% of patients in collagen and PRFM arms, respectively, in a mean time of 6 weeks in both arms, versus 66.67% and 91.67% of patients, respectively, in our study in the mean time of 7–8 weeks for collagen arm and 4–5 weeks for PRFM arm. Impregnation with antibiotics potentially limits the rate of secondary infections.
This study broadly compares the efficacy of treatment options in nonhealing leg ulcers. Limitations of this study include non-homogeneity in patient comorbidities and ulcer profiles limiting a perfect comparison with other studies or drawing inferences about merits/demerits of a particular modality in a specific clinical scenario. A relatively small sample size leading to bias and lack of long-term follow-up are some other limitations.
CONCLUSION
Autologous PRFM resulted in faster healing of chronic leg ulcers when compared with PRP, rhEGF, and collagen particles at 8 weeks, with a shorter mean time to healing of approximately 4–5 weeks with excellent patient acceptability. It is a feasible, relatively simple, and efficacious treatment option for nonhealing leg ulcers.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Not applicable.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Chronic leg ulcers: Epidemiology, aetiopathogenesis, and management. Ulcers. 2013;2013:1-9.
- [Google Scholar]
- Wound healing research: A perspective from India. Int J Low Extrem Wounds. 2005;4:7-8.
- [Google Scholar]
- Use of platelet-rich plasma in the healing of chronic ulcers of the lower extremity. Actas Dermosifiliogr. 2014;105:597-604.
- [Google Scholar]
- A comparative study on therapeutic efficacy of autologous platelet rich fibrin matrix versus zinc oxide and phenytoin paste in non healing ulcers. Indian J Dermatol. 2021;66:620-4.
- [Google Scholar]
- Epidermal growth factor therapy and wound healing: Past, present and future perspectives. Surgeon. 2008;6:172-7.
- [Google Scholar]
- Efficacy of collagen particles in chronic non healing ulcers. J Clin Diagn Res. 2015;9:PC01-3.
- [Google Scholar]
- Autologous platelet-rich fibrin matrix as cell therapy in the healing of chronic lower-extremity ulcers. Wound Repair Regen. 2008;16:749-56.
- [Google Scholar]
- Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care. 2001;24:483-8.
- [Google Scholar]
- Basic studies on the clinical applications of platelet-rich plasma. Cell Transplant. 2003;12:509-18.
- [Google Scholar]
- Autologous platelet-rich fibrin matrix in non-healing trophic ulcers in patients with Hansen’s disease. J Cutan Aesthet Surg. 2017;10:3-7.
- [Google Scholar]
- Autologous platelet rich plasma (PRP) versus leucocyte-platelet rich fibrin (L-PRF) in chronic non-healing leg ulcers--a randomised, open labelled, comparative study. J Evol Med Dent Sci. 2016;5:7460-3.
- [Google Scholar]
- Autologous platelet rich plasma in chronic venous ulcers: Study of 17 cases. J Cutan Aesthet Surg. 2013;6:97-9.
- [Google Scholar]
- Platelet-rich fibrin in nonhealing leg ulcers: A simple and effective therapeutic option. J Cutan Aesthet Surg. 2021;14:160-5.
- [Google Scholar]
- L-PRP/L-PRF in esthetic plastic surgery, regenerative medicine of the skin and chronic wounds. Curr Pharm Biotechnol. 2012;13:1266-77.
- [Google Scholar]
- The type 1 (EGFR-related) family of growth factor receptors and their ligands. Prog Growth Factor Res. 1992;4:1-24.
- [Google Scholar]
- Epidermal growth factor is effective in the treatment of diabetic foot ulcers: Meta-analysis and systematic review. Int J Environ Res Public Health. 2019;16:2584.
- [Google Scholar]
- Efficacy of human recombinant epidermal growth factors vs conventional therapy for the treatment of chronic venous ulcers: A retrospective case series. Wounds. 2021;33:41-9.
- [Google Scholar]
- Epidermal growth factor vs platelet-rich plasma: Activity against chronic wound microbiota. Int Wound J. 2019;16:1408-15.
- [Google Scholar]
- Protease-modulating matrix treatments for healing venous leg ulcers. Cochrane Database Syst Rev. 2016;12:CD011918.
- [Google Scholar]
- Efficacy of autologous platelet rich plasma in comparison with collagen granule dressing in chronic nonhealing ulcer. IJMBS. 2021;5:141-3.
- [Google Scholar]
- A Comparative study of efficacy of antibiotic impregnated collagen granules dressings vs platelet rich fibrin dressings for treatment of trophic ulcers. IOSR J Dent Med. 2021;20:1-4.
- [Google Scholar]





