Translate this page into:
Clinical Uses of NPWT with Irrigation of Normal Saline in Diabetic Foot Ulcer: Outcome Assessed by DEPA Score
Address for correspondence: Dr. Sakshi Goyal, Department of General Surgery, L.N. Medical College and J.K. Hospital, JK Campus, PG1 Hostel, Bhopal, Madhya Pradesh, India. E-mail: sakshigoyal750@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Introduction:
Diabetic foot ulcer (DFU) is a common complication of uncontrolled diabetes. Negative pressure wound therapy (NPWT) with irrigation of normal saline is one of the methods for wound care and dressing techniques in DFU. Wound assessment is another aspect of DFU management for deciding whether the wound is prepared or not for coverage. The present study uses DEPA score as a wound assessment tool in DFU.
Materials and Methods:
This case series include 11 patients with DFU who were treated using NPWT with simultaneous irrigation of normal saline.
Results:
All 11 patients were male and age more than 60. Most patients have duration of diabetes for less than 10 years. Staphylococcus aureus (n = 5, 45.4%) was most common bacterial flora. Most patients in series presented with DEPA score more than 7 and after application of NPWT instillation therapy significant improvement seen with score in most of the patient with DEPA score below 6. Mean time for NPWT (irrigation) application was 15 days. Mean time of wound preparation was 18.7 days. Final surgical procedures executed in all patients, split skin grafting performed in 7 patients. 4 patients had wound coverage by reverse sural flap (2), medial plantar flap (1) and local flap coverage (1).
Conclusion:
NPWT with normal saline irrigation is an effective method of wound preparation in DFU. DEPA score is an important tool for assessment of wound preparation which gives exact information for timing of wound coverage once diabetic foot wound is prepared.
Keywords
DEPA
diabetic foot ulcer
NPWT
VAC
wound healing
INTRODUCTION
Diabetic foot ulcer (DFU) is a common complication of uncontrolled diabetes with annual incidence between 9.1 and 26.1 million worldwide.[1] By definition, DFU is a full-thickness wound which is present at a level distal to the ankle in patients with diabetes.[23] DFU can lead to foot amputation which can be prevented by better understanding of diabetes and DFU. The first step in DFU management is proper assessment and clinical examination of wound. Treatment of DFU starts with control of diabetes followed by debridement and wound care with appropriate dressing and culture-based antibiotic coverage. There are several conventional guidelines of wound care and dressing techniques in DFU.[4] One of them is vacuum-assisted closure (VAC) therapy which promotes healing by applying the specialized dressing over the ulcer bed using the subatmospheric negative pressure. This therapy was alternatively termed as negative pressure wound therapy (NPWT) in a series of patients which was first published 13 years ago.[5] Combining NPWT with irrigation of normal saline cleanses the wound and lessens the bacterial bioburden. Thus it helps in wound bed optimization and is a more effective therapy than VAC alone.[6] Wound assessment is another aspect of DFU management for deciding whether the wound is prepared or not for coverage. The present study uses DEPA score as a wound assessment tool in DFU and attempts to address the benefits of NPWT with irrigation of normal saline in wound bed preparation.
MATERIALS AND METHODS
Place of study
Department of General Surgery, L.N. Medical College and J.K. Hospital Bhopal, Madhya Pradesh.
Type of study
Prospective study
Duration of study
1 year (between January 2019 and December 2019)
Sampling method
Consecutive
Sample collection
11 patients diagnosed with DFU were included in the study. All these patients underwent adequate surgical debridement followed by the application of NPWT with irrigation of normal saline. In all case records of DFU, initial diagnosis was made on the basis of detailed history and clinical examination. Details of patients such as age, sex, and duration of diabetes were recorded. Clinical examination including site, size and specification of DFU according to DEPA score was noted. Other details such as culture and sensitivity of wound swab, time of NPWT application, time for wound preparation, and final surgical procedures were also recorded.
Inclusion criteria
Patients of DFU with controlled diabetes and who underwent surgical debridement followed by NPWT application with NS instillation therapy were included.
Exclusion criteria
The following patients were excluded:
patients with uncontrolled diabetes, peripheral vascular disease, coagulopathy, cardiac disease, stroke, and chronic kidney failure;
patients with any contraindications to NPWT application: suspected malignancy, untreated osteomyelitis, unexplored fistula, sensitivity to silver dressing.
Institutional Ethical approval
Obtained.
Technique
In the present study, the NPWT dressing was applied in all 11 patients. This NPWT dressing (V.A.C. Veraflow; KCI USA, Inc.) consists of a precisely cut sealed sponge over the wound bed connected with a suction pipe, a vacuum pump, and a reservoir. A negative local pressure was applied to the wound in continuous mode from 100 to 125 mmHg.
Patients also received simultaneous irrigation with normal saline per cycle, with 10 min soak time. After this soak time, negative pressure was applied for 3 h 50 min (negative pressure cycle time). This cycle of total 4 h duration repeated six times in a day. NPWT dressing was changed after every 5 days. The endpoint of NPWT was the appearance of healthy granulation tissue with DEPA scores less than 6.
RESULTS
All 11 patients studied were males with diagnosis of infected DFU. Maximum number of patients in this series was above 60 years of age [Table 1]. Most patients have duration of diabetes for more than 10 years. Majority of the patients were found to have Staphylococcus aureus growth in DFU. The mean time for NPWT application was 15 days. Healthy infection-free granulation tissue was obtained in all patients. The mean time of wound preparation, which was calculated from wound debridement to final surgical procedure, was 18.7 days.
| S. no. | Diagnosis (site of DFU) | Age/sex | Duration of DM (years) | Microorganisms isolated (in pus C/S) | Days of NPWT irrigation application | Time of wound preparation (days) | Final surgical procedure |
|---|---|---|---|---|---|---|---|
| 1 | Left heel [Figure 1] | 44/M | 3 | S. aureus | 15 | 17 | Medial plantar flap |
| 2 | Midfoot left side [Figure 2] | 62/M | 8 | E. coli | 25 | 32 | Reverse sural flap |
| 3 | Tendo-achillis region right side [Figure 3] | 60/M | 14 | S. aureus | 10 | 15 | SSG |
| 4 | Dorsum of right foot | 57/M | 5 | S. aureus | 15 | 18 | SSG |
| 5 | Right heel | 35/M | 12 | P. aeruginosa | 10 | 13 | Reverse sural flap |
| 6 | Dorso-lateral aspect of right foot | 61/M | 6 | E. coli | 20 | 24 | SSG |
| 7 | Medial malleolus left foot | 45/M | 16 | P. aeruginosa | 10 | 14 | SSG |
| 8 | Dorsum of right foot | 74/M | 11 | P. mirabilis | 15 | 17 | SSG |
| 9 | Head of first metatarsal right foot | 62/M | 4 | S. aureus | 10 | 13 | Local flap |
| 10 | Dorsum of left foot | 65/M | 9 | E. coli | 15 | 19 | SSG |
| 11 | Dorso-lateral foot lateral malleolus and lower lateral side of leg right side | 51/M | 12 | S. aureus | 20 | 24 | SSG |
| Mean | 9.09 | 15 | 18.7 |
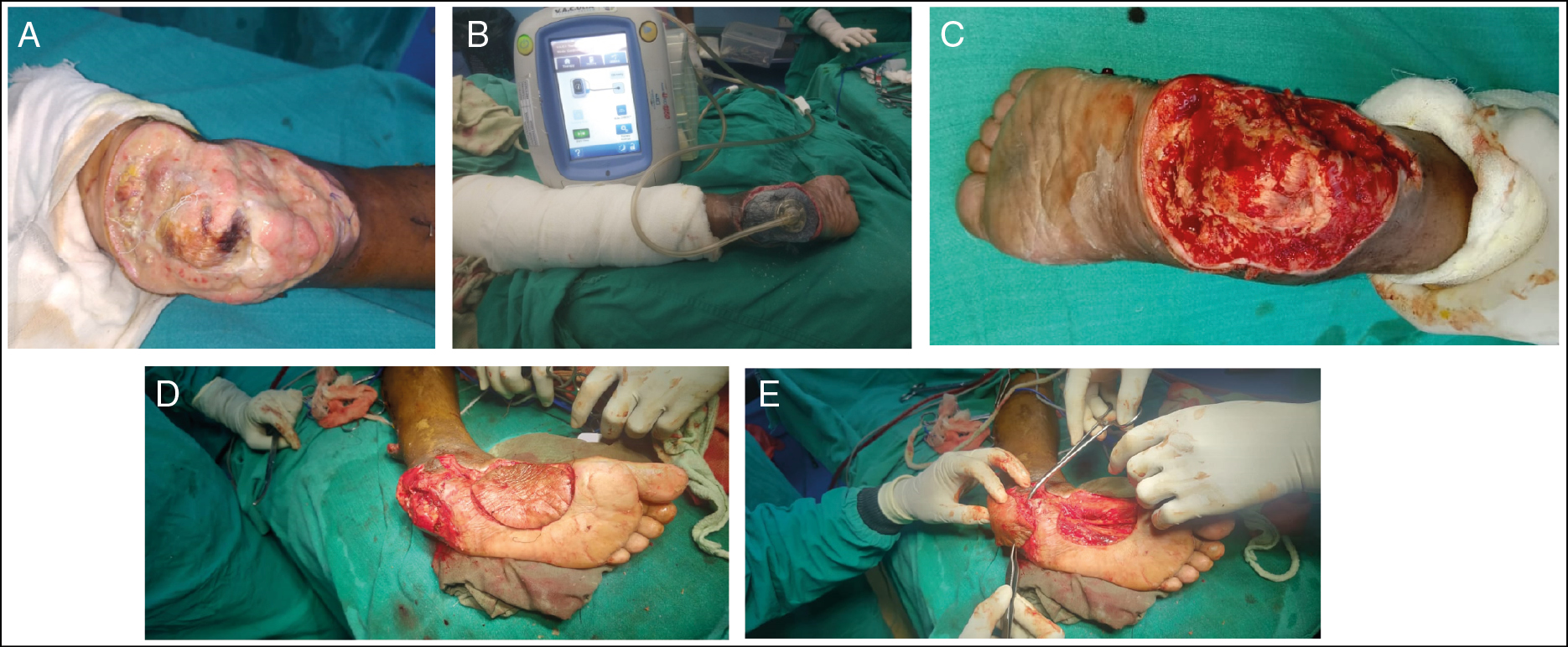
- DFU at left heel. (A) Before NPWT instillation therapy. (B) NPWT instillation therapy. (C) After NPWT instillation therapy. (D) Medial plantar harvesting. (E) Medial plantar flap coverage
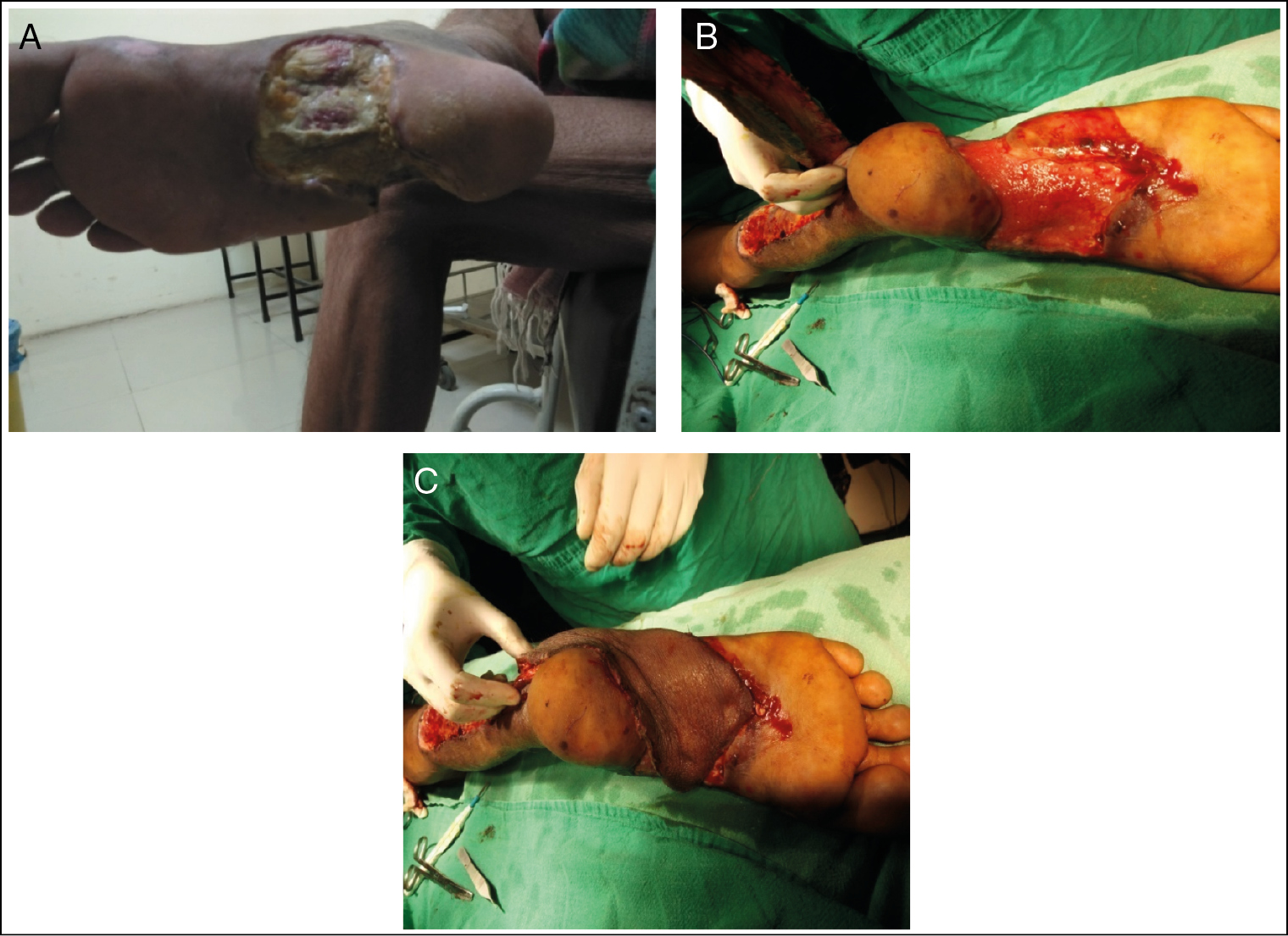
- DFU medial and lateral midfoot. (A) Before NPWT application. (B) After NPWT application. (C) Reverse sural flap coverage
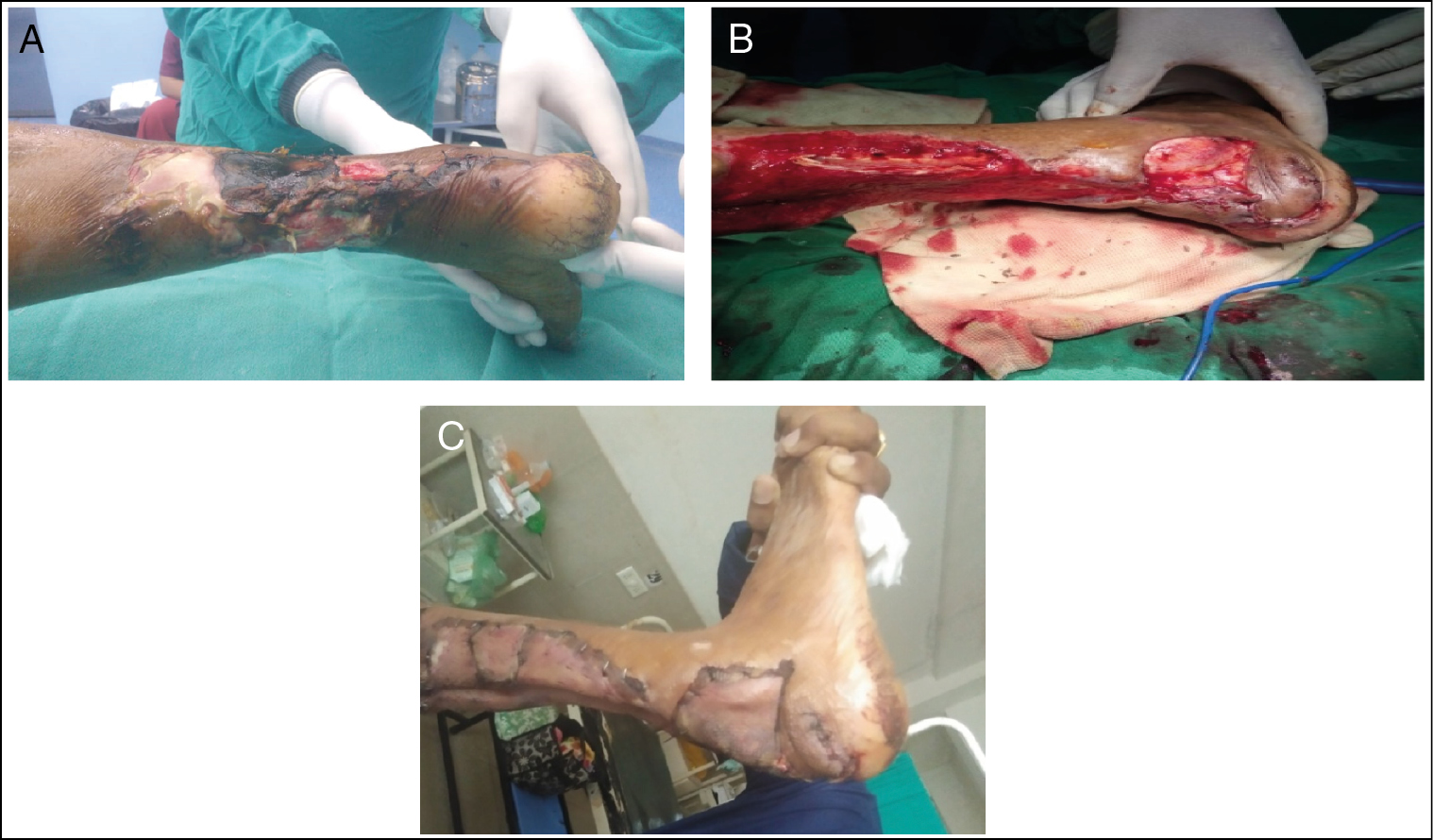
- (A) DFU with necrotizing fasciitis with right tendo-achilles region. (B) After NPWT instillation. (C) Skin grafting over tendo-achilles region
Final surgical procedures were executed in all patients, and split skin grafting was performed in seven patients. Four patients had wound coverage: reverse sural flap (2), medial plantar flap (1), and local flap (1).
We achieved success with coverage of diabetic foot wound after wound preparation by NPWT with normal saline irrigation. No complications related to NPWT were observed. Three patients had a mild local itching complaint, which was successfully treated with oral medication.
DISCUSSION
Diabetes mellitus is a metabolic disease with increasing prevalence in India, which poses significant healthcare and financial burden for patients due to increasing mortality and morbidity because of its complications. At present, India is having the second highest number of people with DM next to China and accounts for almost 1/6th of the diabetic patients in the world, and the prevalence rate of diabetes in India is 2.4% in rural and 12–17% in urban population.[7]
The persistent hyperglycemia in diabetic patients with poor glycemic control leads to micro- and macrovascular complications. DFUs are the most common complications of uncontrolled diabetes mellitus. Most of the patients in this study belonged to the age group of more than 60. Similarly, Saseedharan et al.[8] also suggested male predominance (n=11, 59.7%) in a study on 261 patients, revealing that DFU is more common in old age and male gender.
Most patients (n=6, 54.5%) have duration of diabetes less than 10 years, with mean duration of 9.09 in the present series. Similarly, Jyothylekshmy et al.[9] in a study suggested 56% of patients with duration of diabetes <10 years and mean duration of 8.5 ± 4 years. All 11 patients in our study had controlled diabetic status. Duration of diabetes and diabetic status during treatment have an impact on outcome of DFU management. Antibiotics, offloading, dressings, NPWT therapy, and other modalities of wound management are ineffective if patients have uncontrolled diabetes, so daily monitoring of blood glucose level and judicious use of anti-diabetic therapy are necessary for glycemic control.
Diabetic foot infection is usually present as cellulitis, ulcer, and necrotizing fasciitis. This infection is another common cause of morbidity in patients with DFU, leading to complications such as gangrene and eventually amputation.
Management of DFU includes appropriate culture and sensitivity-based antibiotic coverage with frequent microbiological examination of bacterial flora over the bed of DFU. We performed swab culture and sensitivity of antimicrobial agents test to assess the microbial flora and found that the majority of patients in first wound culture has S. aureus (n=5, 45.4%) growth in DFU. Other bacterial floras that was isolated in the present series were Pseudomonas aeruginosa, Escherichia coli, and Proteus mirabilis. Ramakant et al.[10] studied the bacterial etiology and antibiotic sensitivity pattern of DFU in India in 1632 cultures. The most common pathogens in the first culture were P. aeruginosa (20.1%), S. aureus (17.2%), and E. coli (16.3%).
Debridement forms mainstay treatment for DFU for reducing the bacterial load and desloughing the devitalized tissue and usually performed prior to NPWT application. There are various methods of debridement.[11] Sharp surgical debridement is the most effective and fastest method of debridement. DFU with heavy exudate needs a dressing that absorbs moisture, whereas dry wounds need topical treatments that add moisture. Topical antimicrobial agents as well as antimicrobial impregnated wound dressings might be useful for preventing or treating mild infections. The drawback of conventional dressing is that it is always associated with extended wound preparation time adding discomfort to the patients.
NPWT is an alternative of these conventional dressings which reduces the bioburden of wound. NPWT reduces the wound surface area and promotes granulation tissue formation with added advantage of low complication rate, greater comfort to the medical team and patient, reduced time of hospitalization, reduced use of antibiotics, and number of dressing change.[12131415] Other studies also suggest that NPWT is more effective than these wound dressing and topical antimicrobial.[616]
NPWT with irrigation is a modification of the conventional NPWT therapy, combining the benefits of NPWT with controlled delivery of topical solutions [such as cleansers (normal saline), antiseptics, and antibiotics to the wound bed]. This modification involves the instillation of substances into the sealed wound via an additional tubing system while the vacuum pump is paused. The foam is thus impregnated with the instilled fluid. This process helps; the topical solution comes into contact with the entire area between the foam and the wound surface.[17]
In the present study, the mean time for NPWT (with NS irrigation) application was 15 days, ranging from 10 to 25 days. Apelqvist et al.[18] showed similar results of normal saline with the mean duration of 12 days for NPWT with irrigation, four cycles per day. Additionally, there are clinical observations that NPWT with irrigation by saline is more effective in wound healing than NPWT alone.
NPWT is a very useful therapy, although the cost related to NPWT is a drawback of this excellent therapy and especially the added feature like irrigation with normal saline further increases the cost, so the authors recommended this therapy especially to those patients who can afford this therapy.
The DEPA score was used to analyze the wound status before and after NPWT instillation therapy. This scoring was initially proposed by a Jordan University Hospital (2004) that creates a score according to depth (D), extent of bacterial colonization (E), phase of healing (P), and associated etiology (A). Each component can be scored, according to its severity, from 1 to 3, and a total score ranging from 3 to 12. The outcome measure of healing was significantly associated with higher scores[19] [Table 2].
| DEPA score | 1 | 2 | 3 |
|---|---|---|---|
| Depth of ulcer | Skin | Soft tissue | Bone |
| Extent of bacterial colonization | Contamination | Infection | Necrotizing infection |
| Phase of ulcer | Granulating | Inflammatory | Non-healing |
| Associated etiology | Neuropathy | Bone deformity | Ischemic |
In a study of 84 patients with DFU, Younes and Albsoul[20] suggested that 32 patients had a DEPA score of ≤6, 34 patients had a DEPA score of 7–9, and 18 patients had a DEPA score of ≥10. All patients with DEPA scores ≤6 had excellent healing, whereas only 15% of those with a score of ≥10 had complete healing in <20 weeks. In conclusion, an increasing DEPA score is associated with increased risk of amputation and poor healing. Furthermore, inclusion of the phase of ulcer healing into the DEPA system increases the accuracy of predicting the outcome of DFUs. In the present series, we also found the same results with most of the patients of DFU presented with DEPA scores more than 7 and after application of NPWT instillation therapy, patients showed significant improvement with decrease in DEPA score below 6 [Table 3].
| S. no. | Site of wound | Specificity of ulcers according to DEPA score with size and extent (individual score) | |
|---|---|---|---|
| Before | After | ||
| 1 | Left heel [Figure 1] | Ulcer of size 13 × 9 cm with deep to bone,[3] infection,[2] non healing,[3] diabetic neuropathy[1] | Ulcer of size 12 × 8 cm with deep to bone,[3] contamination,[1] granulating,[1] diabetic neuropathy[1] |
| 2 | Midfoot left side [Figure 2] | Ulcer of size 10 × 8 cm with soft tissue deep,[2] Necrotising fasciitis,[3] non-healing,[3] diabetic neuropathy[1] | Ulcer of size 10 × 7 cm with soft tissue deep,[2] contamination,[1] granulating and healing,[1] diabetic neuropathy[1] |
| 3 | Tendo-achillis region right side [Figure 3] | Ulcer of size 16 × 6 cm with soft tissue involvement,[2] necrotizing fasciitis,[3] non-healing,[3] diabetic neuropathy[1] | Ulcer of size 16 × 3 cm, soft tissue involvement,[2] mild contamination,[1] granulating and healing,[1] diabetic neuropathy[1] |
| 4 | Dorsum of right foot | Ulcer of size 14 × 8 cm with soft tissue involvement,[2] necrotizing fasciitis,[3] non-healing,[3] diabetic neuropathy[1] | Ulcer of size 12 × 6 cm, soft tissue involvement,[2] mild infection,[2] granulating and healing,[1] diabetic neuropathy[1] |
| 5 | Right heel | Ulcer of size 5 × 4 cm with deep to bone,[3] infection,[2] non-healing,[3] diabetic neuropathy[1] | Ulcer of size 5 × 4 cm with deep to the bone,[3] contamination,[1] granulating and healing,[1] diabetic neuropathy[1] |
| 6 | Dorso-lateral aspect of right foot | Ulcer of size 16 × 9 cm with soft tissue involvement,[2] infected,[2] inflammatory,[2] diabetic neuropathy[1] | Ulcer of size 15 × 8 cm with soft tissue involvement,[2] contamination,[1] granulating,[1] diabetic neuropathy[1] |
| 7 | Medial malleolus left foot | Ulcer of size 6 × 5 cm with soft tissue involvement involvement,[2] infected,[2] inflammatory,[2] diabetic neuropathy[1] | Ulcer of size 5 × 4 cm with skin involvement,[1] contamination,[1] granulating,[1] diabetic neuropathy[1] |
| 8 | Dorsum of right foot | Ulcer of size 12 × 8 cm with soft tissue involvement,[2] infected,[2] inflammatory,[2] diabetic neuropathy[1] | Ulcer of size 10 × 8 cm with soft tissue involvement,[2] contamination,[1] granulating,[1] diabetic neuropathy[1] |
| 9 | Head of first metatarsal right foot | Ulcer of size 5 × 4 cm with soft tissue involvement,[2] infected,[2] inflammatory,[2] diabetic neuropathy[1] | Ulcer of size 5 × 4 cm with soft tissue involvement,[2] contamination,[1] granulating,[1] diabetic neuropathy[1] |
| 10 | Dorsum of left foot | Ulcer of size 10 × 6 cm with soft tissue involvement,[2] necrotizing fasciitis,[3] non-healing,[3] diabetic neuropathy[1] | Ulcer of size 9 × 6 cm, soft tissue involvement,[2] mild contamination,[1] granulating and healing,[1] diabetic neuropathy[1] |
| 11 | Dorso-lateral foot Lateral malleolus and lower lateral side of leg right | Ulcer of size 28 × 13 cm soft tissue involvement,[2] infected,[2] non-healing,[3] diabetic neuropathy[1] | Ulcer of size 26 × 12 cm soft tissue involvement,[2] contamination,[1] granulating and healing,[1] diabetic neuropathy[1] |
In the present series, the mean time of wound preparation which was calculated from the time of wound debridement to final surgical procedure was 18.7 days, ranging from 13 to 32 days. A study of Kim et al.[21] on 142 patients compared NPWT instillation and NPWT no-instillation groups and concluded that time to final surgical procedure was significantly shorter for the 6- and 20-min dwell time groups (7.8 ± 5.2 and 7.5 ± 3.1 days, respectively) compared with the no-instillation group (9.23 ± 5.2 days) (P ≤ 0.05).
After wound preparation with granulation over the DFU is done, final surgical procedures were executed. In the present series, split skin grafting is performed in seven patient. Four patients had wound coverage: reverse sural flap (two), medial plantar flap (one) and local flap (one). We achieved success in all the 11 patients with satisfactory coverage of DFU having success in achieving the granulated wound bed for coverage by NPWT with normal saline irrigation. In a study by Zelen et al.,[22] a total of 14 out of 19 (74%) patients healed completely, with a median healing time of 34 days (range 9–114). This success rate is comparable to our result, which further proves the efficacy of NPWT with irrigation.
The drawback of this study is the small sample size with lack of control group, which did not allow direct comparison of NPWT with irrigation feature with conventional NPWT. Further, a large series is needed to prove the efficacy of instillation therapy of NPWT dressing over the NPWT.
CONCLUSION
NPWT normal saline instillation therapy has both economic and clinical advantages in wound bed preparation. Use of this therapy has advantage in faster healing rates, reduced dressing changes, as well as reduced time of wound preparation. Usually, the diabetic foot wound is assessed on the basis of clinical examination; we in our series found that along with clinical examination, DEPA score is an important tool for the assessment of wound preparation which gives exact information for timing of wound coverage once diabetic foot wound is prepared.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Epidemiology of diabetic foot ulcers and amputations: Evidence for prevention. In: Williams R, Herman W, Kinmonth AL, Wareham NJ, eds. The evidence base for diabetes care. Chichester; Hoboken (NJ): John Wiley & Sons, Ltd; 2003. p. :641-65. [DOI: 10.1002/0470846585.ch28]
- [Google Scholar]
- International consensus and practical guidelines on the management and the prevention of the diabetic foot. International Working Group on the diabetic foot. Diabetes Metab Res Rev. 2000;16(Suppl. 1):S84-92.
- [Google Scholar]
- Unnikrishnan AG. Choice of wound care in diabetic foot ulcer: A practical approach. World J Diabetes. 2014;5:546-56.
- [Google Scholar]
- Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann Plastic Surg. 1997;38:563-77.
- [Google Scholar]
- Negative pressure wound therapy after partial diabetic foot amputation: A multicentre, randomised controlled trial. Lancet. 2005;366:1704-10.
- [Google Scholar]
- Park’s textbook of preventive and social medicine. (22nd ed).
- Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Braz J Microbiol. 2018;49:401-6.
- [Google Scholar]
- Epidemiology of diabetic foot complications in a podiatry clinic of a tertiary hospital in South India. Indian J Health Sci. 2015;8:48-51.
- [Google Scholar]
- Changing microbiological profile of pathogenic bacteria in diabetic foot infections: Time for a rethink on which empirical therapy to choose? Diabetologia. 2011;54:58-64.
- [Google Scholar]
- Debridement Procedures for Managing Diabetic Foot Ulcers: A Review of Clinical Effectiveness, Cost-effectiveness, and Guidelines. Canadian Agency for Drugs and Technology in Health; 2014.
- Vacuum-assisted closure: A new method for wound control and treatment: Animal studies and basic foundation. Ann Plast Surg. 1997;38:553-62.
- [Google Scholar]
- Evidence-based medicine: Vacuum-assisted closure in wound care management. Int Wound J. 2007;4:256-69.
- [Google Scholar]
- Negative pressure wound therapy: A systematic review of effectiveness and safety. Eur J Vasc Endovasc Surg. 2008;36:438-48.
- [Google Scholar]
- The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg. 2008;122:786-97.
- [Google Scholar]
- Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: A multicenter randomized controlled trial. Diabetes Care. 2008;31:631-6.
- [Google Scholar]
- Recommendations on negative pressure wound therapy with instillation and antimicrobial solutions—When, where and how to use: What does the evidence show? Int Wound J. 2013;10(Suppl. 1):32-42.
- [Google Scholar]
- Guidelines on the classification of diabetic foot ulcers (IWGDF 2019) Diabetes/Metab Res Rev. 2020;36
- [Google Scholar]
- The DEPA scoring system and its correlation with the healing rate of diabetic foot ulcers. J Foot Ankle Surg. 2004;43:209-13.
- [Google Scholar]
- The impact of negative-pressure wound therapy with instillation compared with standard negative-pressure wound therapy: A retrospective, historical, cohort, controlled study. Plast Reconstr Surg. 2014;133:709-16.
- [Google Scholar]
- A prospective study of negative pressure wound therapy with integrated irrigation for the treatment of diabetic foot ulcers. Eplasty. 2011; 11:e5.
- [Google Scholar]






