Translate this page into:
Comparing the Effect of Ketamine and Lidocaine on Agitation and Pain in Rhinoplasty: A Randomized Clinical Trial
Address for correspondence: Dr. Hesameddin Modir, Department of Anesthesiology, Faculty of Medicine, Vali-Asr Hospital-Arak University of Medical Sciences, Arak, Iran. E-mail: he_modir@arakmu.ac.ir
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
Emergence agitation (EA) is an important clinical problem that occurs during the initial period of recovery from anesthesia. This study aimed to determine the effects of ketamine and lidocaine administered on agitation level, postoperative pain, and hemodynamic changes in adults after rhinoplasty.
Materials and Methods:
Totally 72 patients scheduled to undergo elective rhinoplasty were enrolled in this prospective study. Patients were randomly divided into three groups including control group (n = 24), ketamine group (n = 24), and lidocaine group (n = 24). Twenty minutes before surgery completion, 1 ml saline was administered intravenously to the saline group, while 0.5 mg/kg ketamine or 1.5 mg/kg lidocaine was administered to two other groups. The emergence agitation level of the patients was evaluated using the Richmond Agitation–Sedation Scale just after extubation and in the post-anesthesia care unit (PACU). Postoperative pain was evaluated by Numerical Rating Scale that scored (from 0 to 10) every 10 min until the patients were discharged from PACU.
Results:
There was a significant difference between EA level between ketamine (P = 0.049) and lidocaine (P = 0.019) groups compared to the control group, and there was a significant difference between pain level between the ketamine (P = 0.008) and lidocaine (P = 0.035) groups compared the to control group, while there was no significant difference between the level of agitation (P = 0.922) and level of pain (P = 0.845) after extubation between the ketamine and lidocaine groups.
Conclusion:
Ketamine and lidocaine are highly effective in preventing EA and pain control. Further studies with a greater sample size and longer follow-up period are needed to confirm the current findings.
Keywords
Emergence agitation
ketamine
lidocaine
rhinoplasty
INTRODUCTION
Rhinoplasty is a surgical technique that may be used to address a range of nasal deformities. It is one of the most difficult operations in the field of face cosmetic surgery.[1] After rhinoplasty, emergence agitation (EA) is not rare because of the presence of nasal packing and blood accumulation in the mouth, both of which may cause mucosal irritation that in turn hamper breathing.[2] On the other hand, despite improvements in surgical technique and pain management, inadequate perioperative pain control during nasal surgery remains a major challenge.[3]
EA is an important clinical problem that occurs during the initial period of recovery from anesthesia. EA, including irritability, lack of consciousness, excitement, and continuous crying is common in the short period after emergence from general anesthesia. These conditions can cause respiratory weakness, nausea, vomiting, an increase in blood pressure and heart rate, and an increase in the need for oxygen.[45]
Although EA is not a long-term situation, it involves problems such as removal of the endotracheal tube by the patient, removal of the angiocatheter, bleeding and injury to the patient and staff, and prolonged hospitalization.[567] EA is usually seen in children, but it is commonly seen in adults, especially in some special surgeries such as nose, ear, throat, head, and neck surgeries. Its occurrence is estimated at 21.3% in children.[28] A major risk factor for EA is pain. In comparison to other types of surgery, nasal surgery is significantly associated with higher rates of emergence agitation. Kim et al. reported that emergence agitation can occur 55.4% of the time, and the nasal pack may be the main trigger. In one study, Elsery reported a 68% incidence of EA after nasal surgery.[910] EA can affect the outcome of the surgery. Therefore, preventing EA can make the patient comfortable and improve the healing process and clinical results.[25]
Many drugs are used to treat EA and control pain, which carries the risk of respiratory depression and may pose risks to the patient during the recovery period from anesthesia. Therefore, preventing EA is more important than treating it.[11] Ketamine is a drug that has sedative, amnesia, and analgesia effects. In some studies, the effect of ketamine on the prevention of EA in dental surgeries and tonsillectomy has been reported.[121314] A number of studies have shown that the use of ketamine under general anesthesia reduces the incidence of EA in children, but the number of these studies in adults is limited.[15] Also, lidocaine reduces sore throat after surgery and thus reduces cough and restlessness.[16] Systemic lidocaine has been shown to be an effective adjunct strategy to reduce postoperative pain.[17] According to the studies, administration of a dose of 0.5 mg/kg of ketamine as a bolus or 1.5 mg/kg of lidocaine reduces airway irritation, pain, and restlessness after surgery.[517]
According to the researcher’s knowledge, there are limited studies with conflicting results about the effects of ketamine and lidocaine on EA prevention and pain control after surgery in adults, so the purpose of this study is to investigate the prevalence of EA in adults undergoing rhinoplasty surgery and compare the effect of ketamine and lidocaine on the prevention of EA and pain control.
MATERIALS AND METHODS
Study setting and patients
This double blind (the patient and the recorder’s nurses did not know which treatment or medication were received) randomized control trial was performed from April 2022 to October 2022 [Figure 1]. A total of 72 patients were eligible to enter the study and randomly divided into three groups namely, control (n = 24), ketamine (n = 24), and lidocaine (n = 24). Sample size determination conducted by the statistical methods for sampling in Med Cal software (International Association of Statistical Computing, Seoul, Korea). The minimum sample size required to detect a significant difference using this test should be at least 24 in each group (72 in total), considering type I error (alpha) of 0.05, power (1-beta) of 0.8, the effect size of 0.81, and the two-sided alternative hypothesis (H1).
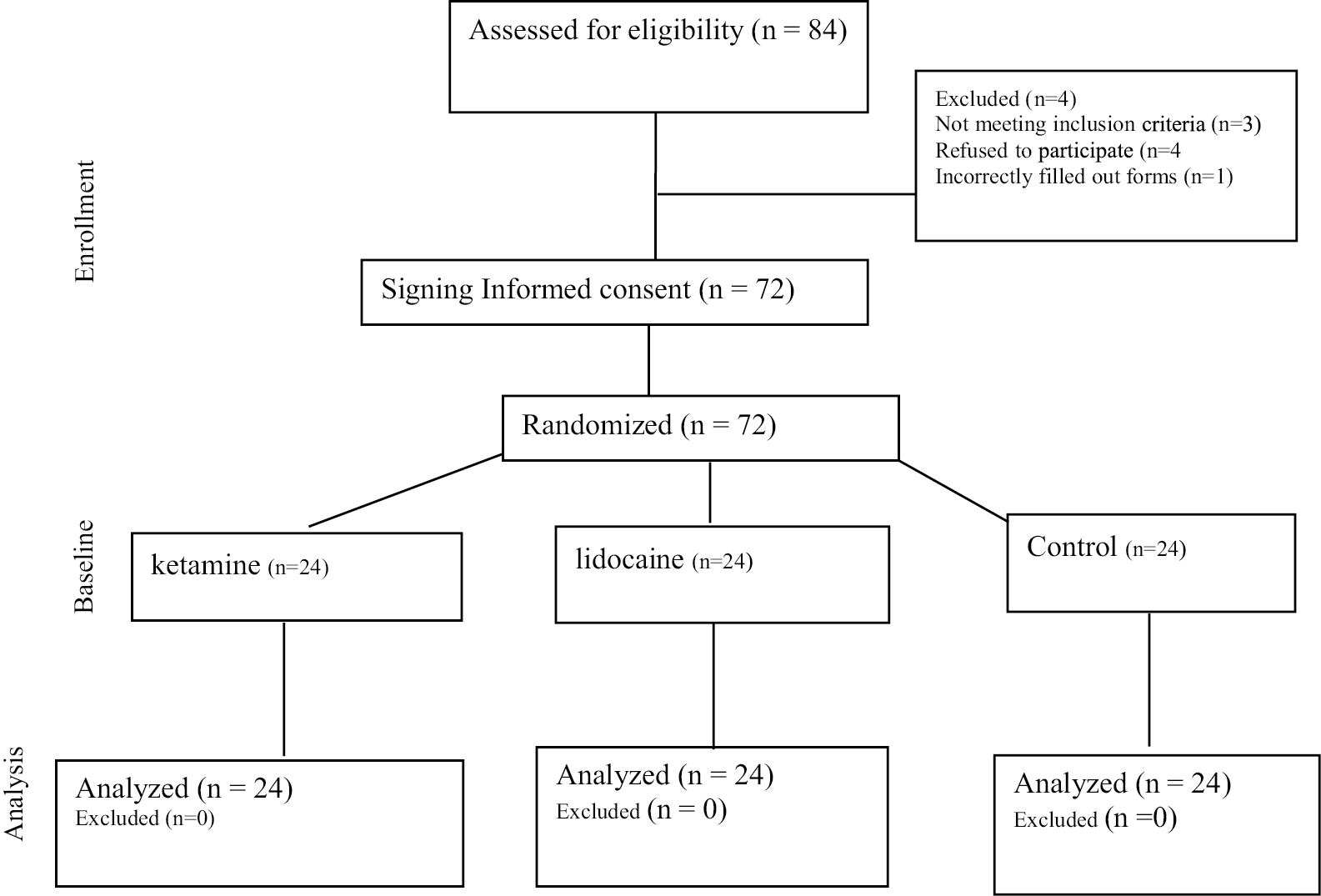
- CONSORT diagram showing the flow of participants through each stage of a randomized trial
Inclusion criteria
Referral for elective rhinoplasty surgery, age 18–45 years, American society of anesthesiologists class classifications I and II, performing surgery with general anesthesia, no use of psychotropic drugs, absence of drug addiction, absence of known sensitivity to the drugs used in the present study.
Exclusion criteria
Taking analgesics and sedatives 24 hours before surgery, history of heart disease, nervous system and neuropsychological disorders, patient with previous rhinoplasty history, history of seizures, duration of anesthesia and surgery more than 2 h.
The method of induction of anesthesia was the same in all three groups: 2 mg/kg of propofol and 2 μg/kg of fentanyl,[5] 0.5 mg/kg of atracurium and 0.03 mg/kg of midazolam. After induction of anesthesia and tracheal intubation, the head of the bed raised 20° to control bleeding.[18] To maintain anesthesia, 100 μg/kg/min of propofol with or without 0.5 monitored anesthesia care isoflurane used along with 50 μg of fentanyl repeated every 30–60 min.[18] Remifentanil infusion used as needed in the form of 1 μg/kg/h to control blood pressure.[19] 20 min before the end of the surgery, patients in the control group were given 1 ml of normal saline, in ketamine group patients received 0.5 mg/kg of ketamine and 1.5 mg/kg of lidocaine in lidocaine group. In all patients, the volume of the injected intervention drug was the same. For reverse of muscle relaxation 0.05 mg/kg neostigmine and 0.01 mg/kg of atropine injected and the patient extubated after the breathing rate is greater than 12 breaths/min and the gag reflex returns.[5] During surgery and until discharge from recovery, blood pressure, heart rate, and percentage of oxygen saturation were recorded every 15–20 min by a trained nurse anesthetist who did not know about the patient group. After extubation and transferring the patient to recovery, the patient’s agitation level was recorded by the RASS scale every 10 min until discharge from recovery by a trained nurse who did not know the patient’s group. The highest RASS scores of the patients were recorded, and patients with a Richmond Agitation–Sedation Scale score of higher than +1 at any time were considered as having EA. If physical control was required in severe EA cases, it was also recorded. Also, after transferring the patient to PACU, postoperative pain recorded by a numerical pain measurement scale (NRS) every 10 min.[5]
Anesthesiologists who recorded the patient’s vital signs and nurses who recorded the RASS and NRS scales were trained by the researcher but did not know the patient group.
Measurements
The emergence agitation level of the patients was evaluated with the Richmond Agitation–Sedation Scale (RASS)[20] just after extubation and on admission to the PACU. This tool contains only 10 items and each item represents one of the levels of consciousness (aggressive state, severe sleepiness, lack of consciousness). The score definitions were as follows: –5, unarousable; –4, deep sedation; –3, moderate sedation; –2, light sedation; –1, drowsy; 0, alert and calm; 1, restless; 2, agitated; 3, very agitated; and 4, combative. The validity and reliability of this tool in The Iranian population have been evaluated and approved by Tadrisi et al.[21]
For postoperative pain evaluation, the Numerical Rating Scale (NRS) was scored (from 0 to 10).[22] Numerical grading of pain intensity is a visual axis that measures pain intensity through questioning patient. This pain scale is used more often. The person grades their pain on a scale of 0 to 10. Zero means “painless” and 10 means “the worst possible pain.” The preference of numerical grading scale of pain intensity compared to visual scale of pain intensity is because it is easier to understand and use. It also shows great credibility in clinical research, especially in patients with less education.[23]
Statistical analysis
The obtained data were analyzed using SPSS version 25 software. Quantitative data are expressed as mean and standard deviation, and qualitative data are expressed as percentage. ANOVA analysis of variance and Tukey’s post hoc test were used to compare the data of three groups. A significance level of 0.05% is considered.
Ethical considerations
Written informed consents were obtained from all study participants. Moreover, approval code was obtained from the Iranian registry of clinical trials (No.: IRCT20220302054164N1). In addition, the ethical committee of Arak University of Medical Sciences approved this project (IR.ARAKMU.REC.1400.281).
RESULTS
A total of 72 patients participated in this study and they were divided into three groups: ketamine, lidocaine, and control. The average age of the participants in the ketamine group was 32.80 ± 6.27 years and 66.7% of the participants were female, in the lidocaine group the average age of the participants was 29.7 ± 7.37 years and 53.3% of the participants were female, and in the control group the average age of the participants was 30.00 ± 5.78 years and 40% of the participants were women. There was no statistically significant difference between the three groups in terms of age (P = 0.457) and gender (P = 0.207) [Table 1].
| Group | Ketamine group | Lidocaine group | Control group | P value | |
|---|---|---|---|---|---|
| Age (mean ± SD) | 32.13 ± 5.78 | 30.50 ± 7.39 | 32.71 ± 5.57 | 0.457 | |
| Gender n (%) | Female | 16 (66.7) | 14 (53.3) | 10 (40.0) | 0.207 |
| Male | 8 (33.3) | 10 (46.7) | 14 (60.0) | ||
There was a statistically significant difference in the average pain score during recovery in the three groups (P = 0.007), based on Tukey’s post hoc test, the average pain score in the ketamine group was lower than the control group (P = 0.008) and in the lidocaine group was less than the control group (P = 0.035). The mean of pain score in the ketamine group was lower than the lidocaine group, but it was not statistically significant (P = 0.845) [Table 2]. In addition, the mean score of emergence agitation in PACU in three groups had a statistically significant difference (P = 0.014), and based on Tukey’s post hoc test, the average score of agitation in the ketamine group was lower than the control group (P = 0.049) and in the lidocaine group was also lower than the control group (P = 0.019). No statistically significant difference was found in the ketamine and lidocaine groups (P = 0.922) [Table 2]. Moreover, Table 3 showed that the incidence of agitation (P = 0.037) and pain (P = 0.025) in PACU is significantly higher in control group than other two interventional groups.
| Group | Ketamine (mean ± SD) | Lidocaine (mean ± SD) | Control (mean ± SD) | P value |
|---|---|---|---|---|
| RASS (agitation score) | 5.60 ± 1.59 | 5.40 ± 1.68 | 4.60 ± 1.95 | 0.014 |
| NRS (pain score) | 2.33 ± 2.09 | 2.73 ± 1.87 | 4.60 ± 1.95 | 0.007 |
| Group | Ketamine n (%) | Lidocaine n (%) | Control n (%) | P value |
|---|---|---|---|---|
| RASS (agitation incidence) | 6 (25) | 7 (29.2) | 13 (54.2) | 0.037 |
| NRS ≥ 3 (pain incidence) | 2 (8.33) | 2 (8.33) | 7 (29.16) | 0.025 |
The repeated measurement test showed [Figure 2] that the trend of mean decrease of HR was significantly different among three groups and the highest decrease in HR occurred in control group (P = 0.001). Nevertheless, the MAP [Figure 3] was not significantly different among three groups (P = 0.653).
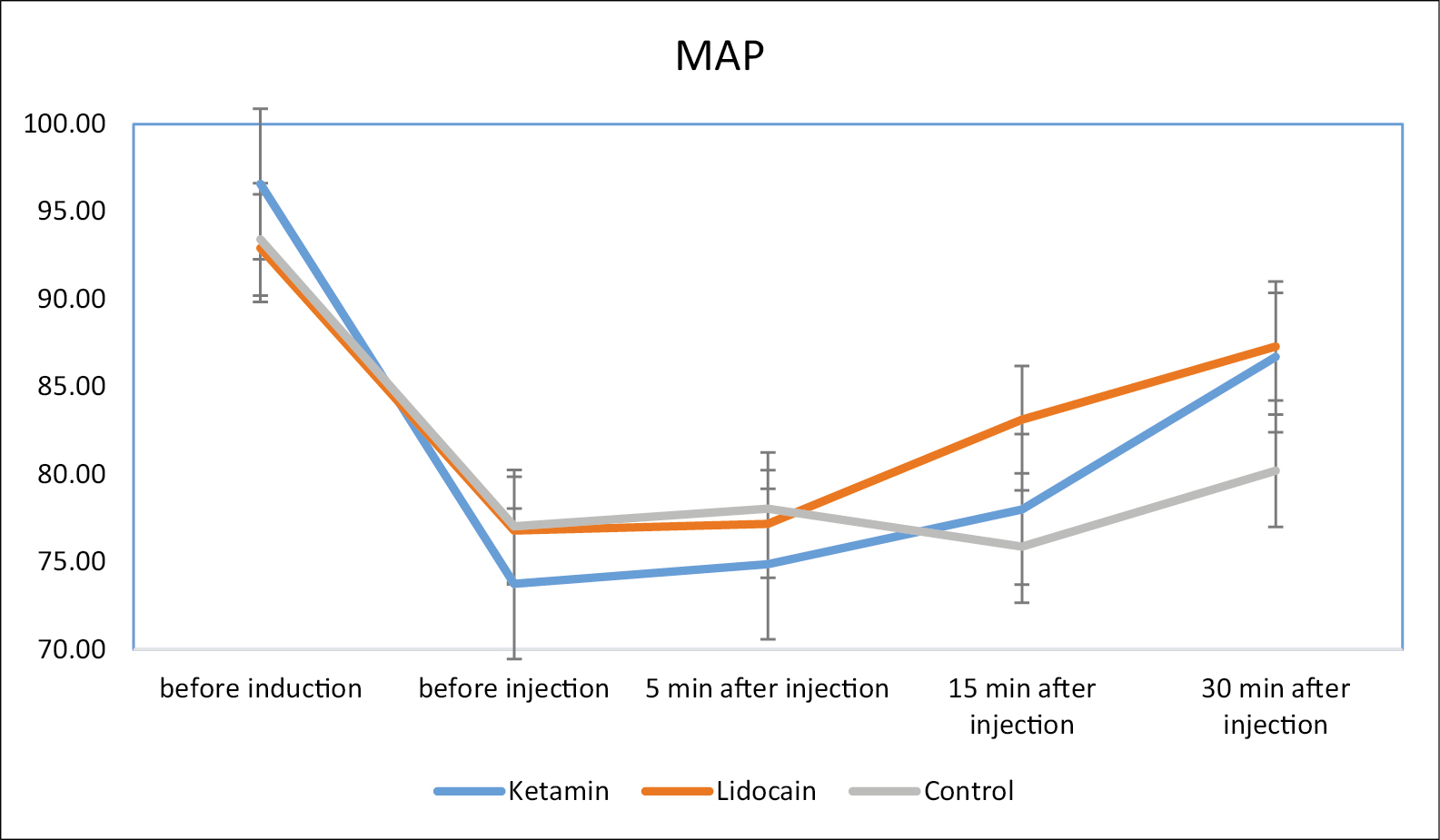
- The repeated measurement trend of mean arterial pressure (MAP) among three study groups
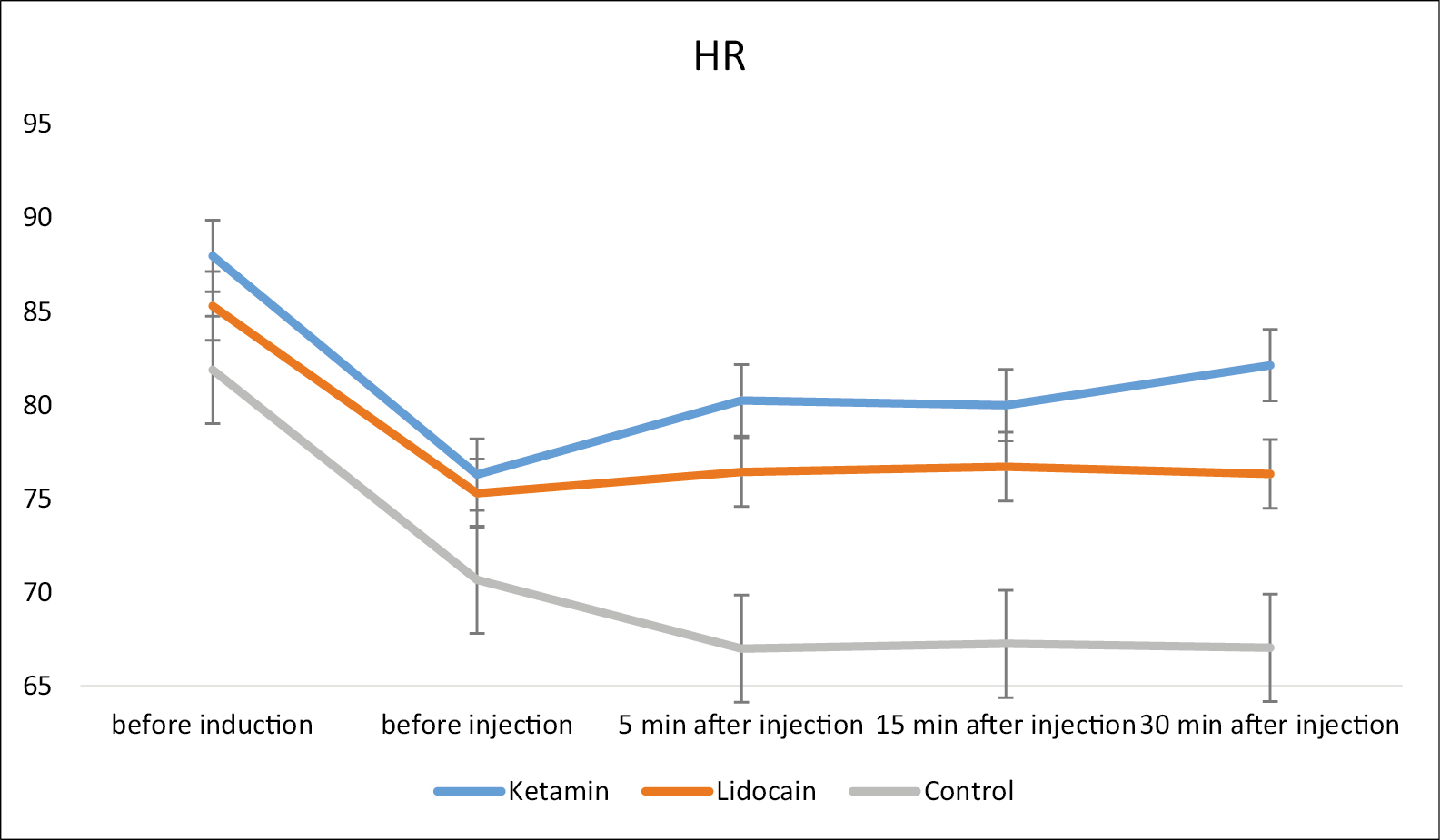
- The repeated measurement trend of heart rate (HR) among three study groups
DISCUSSION
Many factors can contribute to postoperative pain after nasal surgery, such as intraoperative tissue injury, inflammation, nerve stimulation, and swelling. There are many interrelated postoperative events that can trigger agitation, including postoperative pain and hypoxia. As well as causing postoperative complications, pain also causes psychological stress and anxiety, which further aggravates postoperative pain and delays recovery.[17] The occurrence of agitation during recovery from general anesthesia is not uncommon, which can adversely affect postoperative outcomes. Due to nasal osteotomy during rhinoplasty, mild trauma in the early postoperative period could negatively impact the outcome of the procedure. To reduce the trauma risk in such patients, it is essential to prevent EA.[5]
Intravenous lidocaine can cause analgesia in different ways during perioperative periods; it may cause an increase in acetylcholine concentration in cerebrospinal fluid, resulting in the exacerbation of inhibitory descending pain pathways, blocking muscarinic receptors M3, inhibiting glycine receptors, releasing endogenous opioids, reducing inflammation after tissue ischemia, and reducing cytokine production after tissue damage. Lidocaine can also reduce postsynaptic depolarization mediated by N-methyl-D-aspartate receptors.[17] The present study demonstrated a significant decrease in the incidence of emergence agitation in patients who received lidocaine injection compared with those who received saline injection during anesthesia for rhinoplasty. This can be due to the analgesic effect of lidocaine.
During laparoscopic nephrectomy, Tauzin-Fin and Bernard evaluated the effect of adding lidocaine infusion to standard anesthesia protocol. The infusion of lidocaine was continued for 24 h postoperatively and significantly reduced morphine consumption and postoperative pain scores.[24]
A study conducted by Jang and Oh found that 1.5 mg/kg of intravenous lidocaine 5 min before extubation did not decrease the incidence of EA and the severity of postoperative pain after sevoflurane anesthesia.[25] Therefore, our results indicate that 1.5 mg/kg intravenous lidocaine 20 min before extubation leads to a significantly lower EA by preventing physiologic deterioration and pain reduction related to anesthesia and surgery. According to Lee et al. systemic infusion of lidocaine during bimaxillary surgery reduces postoperative pain and analgesic consumption and relieves swelling of the face.[26] Based on our results, the pain score was higher in the control group than in the lidocaine group, similar to those reported by Kim et al. Compared to the control group, lidocaine administered intravenously during thyroid surgery improved postoperative recovery as measured by QoR-40 in female patients.[27] During outpatient laparoscopy, lidocaine improves postoperative recovery with less opioid consumption.[28]
Compared with the control group in our study, perioperative lidocaine use resulted in lower RASS and NRS scores. This may be a result of the pharmacological effects of lidocaine on inflammation, analgesic requirement, and nausea/vomiting.[26]
In this study, we evaluated and compared the effects of ketamine and lidocaine on postoperative emergence agitation and pain in patients who underwent rhinoplasty. We determined that in the ketamine group, NRS and RASS scores were significantly better with less analgesic requirement compared to the control group. EA incidence in the PACU were statistically significantly lower in the ketamine group than control group and the number of patients who required physical control or medications for agitation was also significantly lower in the ketamine group than control. Patients in lidocaine group had better RASS score than ketamine group. On the other hand, ketamine demonstrated better pain control than lidocaine but these differences were not statically significant.
In high doses, ketamine is analgesic, but at sub-anesthetic doses, it is hypnotic. According to previous studies, ketamine significantly reduced the risk of EA when compared to placebo.[29] However, most of these studies were conducted on pediatric patients.[5] A recent study by Lin et al.[30] Found that ketamine combined with butorphanol significantly reduced the incidence of EA among 150 adults operated on for gastric cancer. In the present study, we report one of the first studies in the literature to show that sub-anesthetic doses of ketamine injection significantly reduce EA incidence just after extubation and during post-operative follow-ups in PACUs. The results of our study are consistent with those of Demir.[5]
In our study, EA incidence was determined to be as high as 73.3% in control group, 33.3% in lidocaine group and 26.6% in ketamine group during in the PACU. These higher incidence rates may be associated with the characteristics of rhinoplasty that cause hampered breathing during the early postoperative period. Based on numerical pain rating scale none of the patients reported NRS > 3. In lidocaine group 6.7%, in ketamine group 6.7% and 26.7% in control group reported NRS = 3, which means postoperative mild pain was significantly higher in control group (P < 0.05). Studies regarding ketamine and lidocaine administration versus other therapies for EA prevention and pain control are limited.
According to Kim et al., ketamine was more effective than midazolam in preventing EA in children following sevoflurane anesthesia.[31] In children undergoing adenotonsillectomy after sevoflurane anesthesia, Hadi et al. reported that intraoperative low-dose intravenous ketamine followed by dexmedetomidine significantly reduced postoperative pain and EA.[32] Another study found that intravenous administration of ketamine 0.5 mg/kg or fentanyl 1 micg/kg before tonsillectomy significantly reduced postoperative agitation in sevoflurane-anesthetized children with tonsillectomy without any differences between the two medications.[33]
In a previous study, ketamine administration failed to prevent the occurrence of EA after inhalation anesthesia in children subjected to caudal blocks, and pain was identified as the primary cause.[34] In our study, the pain score was found to be low in both ketamine and lidocaine groups. The prevalence of NRS = 3 score was significantly higher in control group than in study groups at the time of admission to the PACU. A low NRS score at the time of admission to the PACU may be due to the analgesic effect of ketamine and lidocaine. Therefore, the difference between the groups in terms of the incidence of EA can be attributed to the low postoperative pain levels in study groups.
The present study has some limitations. In the postoperative period, all patients were followed only until discharge from the PACU. Furthermore, 72 patients were included in the study; although the number of patients included in the study was not small, a larger sample size might have revealed a difference between the groups in terms of the incidence of EA and pain control. On the other hand, the lack of data regarding the comparison of ketamine and lidocaine with other analgesics or anesthetics is the main limitation of the present study.
CONCLUSION
The intraoperative administration of ketamine and lidocaine significantly reduce the incidence of EA and enhanced pain control following rhinoplasty. In surgeries where post-operative EA and pain management is essential, the administration of a low dose of 0.5 mg/kg ketamine or 1.5 mg/kg lidocaine is similarly effective and does not have any negative effects on hemodynamics.
Financial support and sponsorship
This study was supported by Arak University of Medical Sciences.
Conflicts of interest
There is no conflict of interest.
Consent to participate
All the authors are consent to participate and publishing article in this journal.
Acknowledgements
This article is the result of a thesis in general medicine. Hereby, we would like to extend a special debt of gratitude to the Valiasr Hospital’s clinical research council for its assistance and guidance and to thank the research deputy of Arak University of Medical Sciences for his contributions and support throughout the development of this study.
REFERENCES
- Adding ketamine to xylocaine and epinephrine in local infiltration for rhinoplasty, postoperative analgesia and side effects. Egypt J Hosp Med. 2021;85:3448-53.
- [Google Scholar]
- Evaluation of emergence agitation after general anaesthesia in rhinoplasty patients: inhalation anaesthesia versus total intravenous anaesthesia. Am J Otolaryngol. 2020;41:102387.
- [Google Scholar]
- Perioperative analgesia for patients undergoing septoplasty and rhinoplasty: an evidence-based review. Laryngoscope. 2019;129:E200-12.
- [Google Scholar]
- Risk factors of emergence agitation in adults undergoing general anesthesia for nasal surgery. Clin Exp Otorhinolaryngol. 2015;8:46-51.
- [Google Scholar]
- Prevention of emergence agitation with ketamine in rhinoplasty. Aesthetic Plast Surg. 2018;42:847-53.
- [Google Scholar]
- A comparative study of emergence agitation between sevoflurane and propofol anesthesia in adults after closed reduction of nasal bone fracture. Korean J Anesthesiol. 2012;63:48-53.
- [Google Scholar]
- Knowledge and attitude of nonpsychiatric physicians regarding suicide in spinal cord injury patients and need for structured psychiatric education for suicide prevention: a prospective survey pilot study. Medicine (Baltimore). 2019;98:e14901.
- [Google Scholar]
- Emergence agitation in adults: risk factors in 2,000 patients. Can J Anaesth. 2010;57:843-8.
- [Google Scholar]
- A randomised controlled trial comparing rocuronium priming, magnesium pre-treatment and a combination of the two methods. Anaesthesia. 2012;67:748-54.
- [Google Scholar]
- Post-operative agitation in adults, factors, possible mechanisms and prevention. Anaesthesiol Clin Sci Res. 2017;1:1-3.
- [Google Scholar]
- Effect of ketamine on emergence agitation following septoplasty: a randomized clinical trial. Braz J Anesthesiol. 2021;71:381-6.
- [Google Scholar]
- The effect of ketamine on the incidence of emergence agitation in children undergoing tonsillectomy and adenoidectomy under sevoflurane general anesthesia. Korean J Anesthesiol. 2010;58:440-5.
- [Google Scholar]
- Ketamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesia. Paediatr Anaesth. 2007;17:846-50.
- [Google Scholar]
- Sevoflurane-emergence agitation: effect of supplementary low-dose oral ketamine premedication in preschool children undergoing dental surgery. Eur J Anaesthesiol. 2010;27:353-8.
- [Google Scholar]
- Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can J Anaesth. 2013;60:385-92.
- [Google Scholar]
- Effect of esmolol and lidocaine on agitation in awake phase of anesthesia among children: A double-blind, randomized clinical study. Chin Med J (Engl). 2019;132:757-64.
- [Google Scholar]
- Effects of lidocaine infusion on quality of recovery and agitation after functional endoscopic sinus surgery: randomized controlled study. Open J Anesthesiol. 2020;10:435-48.
- [Google Scholar]
- Comparing labetalol and nitroglycerine on inducing controlled hypotension and intraoperative blood loss in rhinoplasty: a single-blinded clinical trial. Anesth Pain Med. 2017;7:e13677.
- [Google Scholar]
- Comparing remifentanil, magnesium sulfate, and dexmedetomidine for intraoperative hypotension and bleeding and postoperative recovery in endoscopic sinus surgery and tympanomastoidectomy. Med Gas Res. 2018;8:42-7.
- [Google Scholar]
- The richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338-44.
- [Google Scholar]
- Richmond agitation-sedation scale validity and reliability in intensive care unit adult patients; Persian version. Iran J Crit Care Nurs. 2009;2:15-21.
- [Google Scholar]
- Do 0-10 numeric rating scores translate into clinically meaningful pain measures for children? Anesth Analg. 2011;112:415-21.
- [Google Scholar]
- Predicting the intensity of pain in patients with chronic pain based on alexithymia: the mediating role of the behavioral inhibition system. Iran J Psychiatry Behav Sci. 2019;25:56-71.
- [Google Scholar]
- Benefits of intravenous lidocaine on post-operative pain and acute rehabilitation after laparoscopic nephrectomy. J Anaesthesiol Clin Pharmacol. 2014;30:366-72.
- [Google Scholar]
- Intravenous lidocaine does not reduce emergence agitation or pain after sevoflurane anesthesia in children. Korean J Anesthesiol. 2005;49:S14-S19.
- [Google Scholar]
- Intravenous lidocaine for effective pain relief after bimaxillary surgery. Clin Oral Investig. 2017;21:2645-52.
- [Google Scholar]
- Intravenously administered lidocaine and magnesium during thyroid surgery in female patients for better quality of recovery after anesthesia. Anesth Analg. 2018;127:635-41.
- [Google Scholar]
- Systemic lidocaine to improve postoperative quality of recovery after ambulatory laparoscopic surgery. Anesth Analg. 2012;115:262-7.
- [Google Scholar]
- Preventing emergence agitation using ancillary drugs with sevoflurane for pediatric anesthesia: a network meta-analysis. Mol Neurobiol. 2017;54:7312-26.
- [Google Scholar]
- Effect of ketamine combined with butorphanol on emergence agitation of postoperative patients with gastric cancer. Ther Clin Risk Manag. 2016;12:713-7.
- [Google Scholar]
- Comparison of effects of intravenous midazolam and ketamine on emergence agitation in children: randomized controlled trial. J Int Med Res. 2016;44:258-66.
- [Google Scholar]
- The effect of KETODEX on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane based-anesthesia. Int J Pediatr Otorhinolaryngol. 2015;79:671-6.
- [Google Scholar]
- Effect of ketamine versus alfentanil following midazolam in preventing emergence agitation in children after sevoflurane anaesthesia: a prospective randomized clinical trial. J Int Med Res. 2014;42:1262-71.
- [Google Scholar]
- Effects of ketamine and midazolam on emergence agitation after sevoflurane anaesthesia in children receiving caudal block: a randomized trial. Br J Anesthesiol. 2014;64:377-81.
- [Google Scholar]






