Translate this page into:
Persistent lip nodules after hyaluronic acid filler injection
*Corresponding author: Alison Bruce, Department of Dermatology, Mayo Clinic, Jacksonville, Florida, United States. bruce.alison@mayo.edu
-
Received: ,
Accepted: ,
How to cite this article: Pincelli T, Li H, Bruce A. Persistent lip nodules after hyaluronic acid filler injection. J Cutan Aesthet Surg. doi: 10.25259/jcas_151_23
Dear Editor,
Hyaluronic acid (HA) filler adverse effects most frequently include local site reaction, infection, and erythema, which are typically self-limiting. In <1% of cases, fillers may leave persistent nodules that can last months to years after injection.1 Diagnosis can be challenging as patients may be asymptomatic and not relate their development with filler treatment performed several years prior. We describe a case of lip nodules persisting for 5 years after HA filler injection without any inflammatory reaction.
A 57-year-old female with a history of lung transplantation presented with persistent lip nodules for 5 years. She reported a history of Juvéderm (AbbVie Inc., Illinois, USA) lip fillers to that area 5 years prior. Three smooth nodules measuring 0.4 cm each were present on the inner mucosal surface of the upper lip [Figure 1a]. A punch biopsy from one of the nodules demonstrated foreign body material consistent with HA [Figure 1b]. No inflammatory infiltrates or granuloma formation were observed surrounding the HA deposits on histopathology. Small superficial incisions were performed on the upper mucosal lip in the areas overlying the nodules, and manual expression and drainage of the filler were attempted [Figure 1c]. Small amounts of fluid and clear material were released. Following this, each nodule was injected with intralesional hyaluronidase injections (150 U/mL) in 0.1 mL aliquots, for a total of 80 U (0.5 mL). This led to partial resolution of the nodules, and the patient was recommended additional hyaluronidase injections, which she has not yet received.
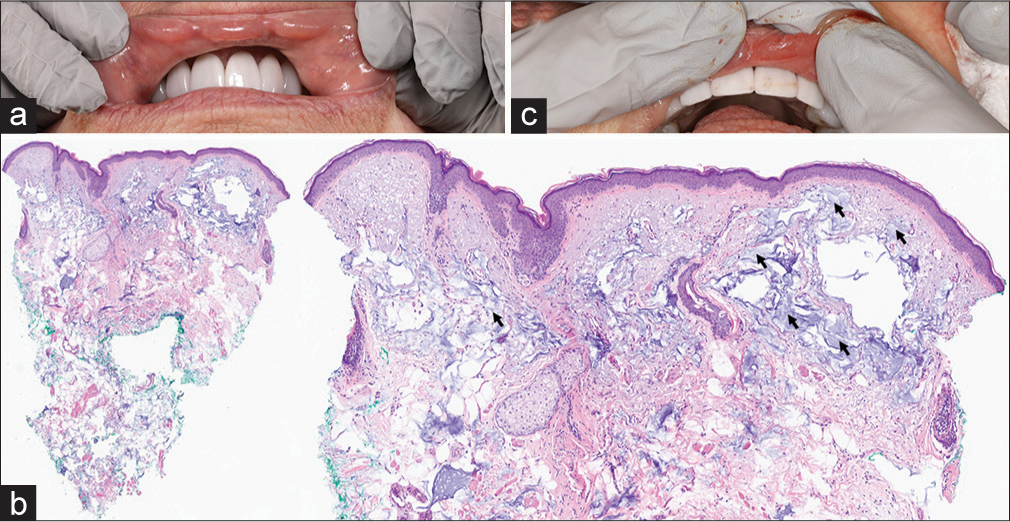
- (a) Lip nodules before treatment. (b) Histopathology demonstrating dermis with superficial filler deposition (black arrows) and absence of granulomatous infiltrate. (c) Nodule treatment with manual expression.
The development of nodules after filler treatment can be categorized as inflammatory or non-inflammatory in origin.2 Non-inflammatory nodules usually occur immediately after treatment and are generally caused by improper placement of filler material. Inflammatory nodules may occur days to years after filler injection and are secondary to a host response to a foreign material. Biodegradable HA, a polysaccharide present in nearly all species, triggers a minimal foreign body response when compared to permanent fillers such as silicone.3
However, prior studies have reported inflammatory nodule formation from HA injection, particularly on the lips, with incidence estimated as 0.4%.4 This may result from biofilm formation following bacterial inoculation during injection. These biofilms resist hyaluronidase and can induce a very low-grade infection with negligible host response.5 In our case, pathologic examination of one of the nodules confirmed the presence of HA years following treatment, indicating failure in the degradation process. Although the patient’s immunosuppressed state may have reduced immune response, it also increased the risk of persistent subclinical infection and biofilm formation. In the present case, a possible explanation for the development of persistent lip nodules includes immunosuppression in the setting of the patient’s known lung transplant, which may have kept the immunogenicity of her HA at a minimum; the formation of a biofilm was associated with minimal immune response, keeping the HA “protected” from degradation by natural hyaluronidases for several years. Our case highlights the importance of a thorough history in diagnosing delayed-onset nodules, particularly in patients with asymptomatic, persistent nodules developing years after initial filler injection.
Authors’ Contributions
Thais Pincelli contributed to study conception, design, data collection, and manuscript writing. Han Li contributed to study analysis and manuscript writing. Alison Bruce contributed to study conception and supervision. All authors provided critical review of study contents and approved the final manuscript.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Inflammatory nodules following soft tissue filler use: A review of causative agents, pathology and treatment options. Am J Clin Dermatol. 2013;14:401-11.
- [CrossRef] [Google Scholar]
- Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Dermatol Surg. 2009;35:1612-9.
- [CrossRef] [Google Scholar]
- Incidence and treatment of delayed-onset nodules after VYC filler injections to 2139 patients at a single Canadian clinic. J Cosmet Dermatol. 2022;21:2379-86.
- [CrossRef] [Google Scholar]
- Probable biofilm formation in the cheek as a complication of soft tissue filler resulting from improper endodontic treatment of tooth 16. Int J Nanomedicine. 2012;7:1441-7.
- [CrossRef] [Google Scholar]





