Translate this page into:
Platelet-rich Fibrin versus Platelet-rich Plasma: A Study to Assess Efficacy as a Regenerative Medicine Strategy for Chronic Cutaneous Ulcers
Address for correspondence: Dr. Arun C. Inamadar, Department of Dermatology, Venereology & Leprosy, Shri B M Patil Medical College, Hospital & Research Centre, BLDE (Deemed to be University), Vijayapura 586103, Karnataka, India. E-mail: aruninamadar@gmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
The management of nonhealing ulcers has been a major challenge clinically. Current therapies include debridement, offloading, etc., which show a poor response. Newer modalities include stem cells, platelet-derived growth factors, and fibrin glues, which reduce healing time. Platelets play a major role in wound healing through the secretion of growth factors, chemokines, etc. and have been an area of interest as a modality in regenerative medicine.
Aims and Objective:
The aim was to study the comparative efficacy of autologous platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) as a regenerative medicine strategy for chronic cutaneous ulcers.
Materials and Methods:
Forty-four ulcers of duration greater than six weeks were enrolled for a comparative study comprising two groups, each divided either into group A receiving PRF dressings or group B receiving PRP dressing for six weeks. The ulcer evaluation was performed at baseline, each weekly dressing, and a two-week follow-up.
Results:
Primary efficacy was assessed by the percentage reduction in the volume of ulcers and re-epithelization at eight weeks. In total, 95.2% of ulcers in group A and 90.4% of ulcers in group B showed complete re-epithelization. One ulcer in group A and two ulcers in group B developed an infection. The recurrence of the ulcer was seen in four ulcers in the PRF group and three ulcers in the PRP group.
Conclusion:
Dressings done with PRF and PRP showed similar efficacy in the percentage reduction in the volume and re-epithelization of chronic cutaneous ulcers. Both dressings were associated with similar complications. PRF and PRP dressings provide a safe, efficacious, and inexpensive regenerative medicine strategy in the healing of chronic cutaneous ulcers.
Keywords
Chronic cutaneous ulcers
platelet-rich fibrin
platelet-rich plasma
INTRODUCTION
An ulcer is a breach in the continuity of skin, epithelium, or mucous membrane caused by sloughing out of inflamed necrotic tissue.[1] Chronic ulcers are formed because of the failure in the orderly process that produces anatomic and functional integrity.[2] Ulcers are considered chronic if they show no tendency to heal after six weeks of appropriate treatment or those that have not fully healed after 12 months.[23] Repeated trauma, poor perfusion or oxygenation, and/or excessive inflammation contribute to the causation and perpetuation of chronicity of ulcers.[1] Thus, the management of these ulcers is a major clinical challenge. Current therapies include debridement, offloading, etc.[1] However, the response to treatment is often poor, and the outcome is disappointing. These wounds place the limb at the risk of infection and amputation and also puts the patients at risk of mortality.[1] Newer modalities of treatment include the use of stem cells, platelet-derived growth factors (PDGFs), fibrin glues, etc., which increase the response in healing chronic wounds.[2]
Blood platelets play a major role in the initiation of cutaneous wound healing.[2] Autologous platelet-rich plasma (PRP) is a platelet suspension in plasma derived from whole blood that is increasingly being used in clinical practice for the treatment of acne scars, chronic ulcers, etc. The concentration of platelets in PRP is two to six folds higher than that of whole blood.[4] Platelet-rich fibrin (PRF) is a new member of platelet concentrates developed by Choukroun et al.[5] It is classified as one of the four families of platelet concentrates, as PRF is a cross between fibrin glue and classic platelet concentrates. Platelet and growth factors are theoretically trapped in the fibrin clot, as platelets are not measured, and growth factors in the exudate are well below the other PRP preparations.[6]
Currently, PRP and PRF are being used widely for many purposes without side effects. Because of the lack of sufficient literature, our study aimed to compare the efficacy of autologous PRF versus PRP as a regenerative medicine strategy for chronic cutaneous ulcers.
MATERIALS AND METHODS
The study was a hospital-based randomized comparative study with patients of age >18 years with cutaneous ulcers greater than six weeks of duration and a size ranging between 0.5 and 10 cm, having a normal platelet count and hemoglobin >10 gm%. Exclusion criteria included infected ulcers and ulcers less than six weeks old. A detailed history of onset, duration, past treatment with topical medications and surgical interventions, and preexisting medical conditions such as hypertension, diabetes, history of Hansen’s disease, venous insufficiency, etc. was recorded. An initial clinical examination was done to determine the size, volume, and condition of ulcers, and findings were recorded in the pro forma and informed consent with clinical photographs was taken. The ulcers were randomized by allocating alternately into two groups: ulcers in group A were treated with PRF dressing weekly and those in group B were treated with PRP dressing weekly.
All ulcers were measured using calipers and cotton-tipped applicators to determine the length, width, and depth of the ulcer. The ulcer volume was calculated using the formula for an ellipse, as an ellipse is closer to a wound shape than a square or rectangle:[789]
Volume of ulcer = (Length × Width × 0.7854) × Depth
The ulcer site was examined and cleaned with an antiseptic solution, if required, paired, and then prepared for PRF/PRP dressing.
PRF was prepared following the protocol developed by Choukroun et al. using a table centrifuge (Remi R-8C, Remi Electrotechnik Ltd., Mumbai, Maharashtra, India) and collection kit (20-mL syringe, 5-mL blood collection tubes). Blood was collected by venipuncture under aseptic precautions in four sterile collection tubes of 5-mL capacity without anticoagulant for every 4 cm[3] of the ulcer. The tubes were placed at opposite sites in a centrifugal machine at 3000 rpm (704.34 g/radius of 7 cm) for 10 min and immediately centrifuged.[5] A well-structured and resistant fibrin clot is thus formed in the middle of the tube, just between the red corpuscles at the bottom and straw-colored acellular plasma at the top. The upper straw-colored layer was discarded, and the middle fraction was collected and cut at the erythrocyte zone as close as possible to the fibrin clot (2 mm below the lower dividing line), where platelets trap massively in the fibrin meshes.[2] A two-stage centrifugation process (double-spin method) was employed for the preparation of PRP.[4] The first spin was at 3000 rpm for 7 min after which the lower red blood cell portion was discarded, and the supernatant containing platelet-poor plasma and buffy coat was centrifuged again at 4000 rpm for 5 min (second spin). The lower one-third of this solution provided approximately 2 mL of autologous PRP for dressing.[4]
In group A, PRF was used to cover the floor of the ulcer, and in group B, PRP was injected into the margin of the ulcer. The ulcer was further dressed with a nonadherent dressing. Dressings were changed weekly in both groups for a maximum of six weeks or until complete re-epithelization depending on the healing response. The ulcer evaluation was performed at baseline and then every week until eight weeks by an investigator for ulcer area, volume, characteristics exudates (presence, color, amount, odor), necrotic tissue and granulation tissue, pain, infection, and re-epithelization. Patient satisfaction and general perception regarding the treatment were also noted. Clinical photographs were taken in identical settings and lighting at every follow-up before successive dressing. Any adverse effects related to therapy were recorded in the pro forma immediately at each sitting. At the end of eight weeks, the final response was evaluated according to the above-mentioned procedure. Patients in both groups were asked to mark their response on a 10-inch-long visual analog scale (VAS). Statistical analysis was done using the Kolmogorov–Smirnov test, and if P value was <0.05, then the results were considered to be statistically significant.
RESULTS
Statistical data from the study were tabulated and analyzed [Table 1].
| Data | Total | PRF | PRP | P | Kolmogorov-Smirnov Z |
|---|---|---|---|---|---|
| Sex distribution | |||||
| Male | 35.7% | 38.1% | 33.3% | 0.747 | |
| Female | 64.2% | 61.9% | 66.7% | ||
| Mean age | 46.2 ± 20.8 years | 48.7 ± 19.1 years | 0.983 | 0.463 | |
| Most common age groups with ulcers | 21–30 years 41–50 years |
21–30 years 51–60 years |
0.551 | ||
| Diagnosis | |||||
| Hansen’s disease | 71.4% | 76.2% | 0.726 | ||
| Non-Hansen’s disease | 28.6% | 23.8% | |||
| Site—most common | Lower limbs | 57.14% | 42.8% | ||
| Mean volume | 1332.7 ± 1266.6 mm3 | 1090.8 ± 1655.4 mm3 | 0.358 | 0.926 | |
| Mean number of dressings | 3.5 ± 1.5 | 3.2 ± 1.2 | 0.983 | 0.463 | |
| Mean percentage reduction in volume of ulcer | 44.8%–71.4% | 46.1%–73.8% | 0.138–0.925 | 0.548–1.156 | |
| Mean VAS | 9.2 ± 1.6 | 8.9 ± 0.9 | 0.551 | ||
| Re-epithelization | 95.2% | 90.4% | |||
| Complications—infection | 4.8% | 9.5% | 0.549 | ||
| Recurrence | 19% | 14.3% | 0.788 |
In our study, the most common cause for chronic ulcers in both groups was Hansen’s disease (73.8%), i.e., 71.4% in the PRF group and 76.2% in the PRP group, respectively. Lower limb ulcers were most commonly seen in both groups with the right foot being predominantly involved in both groups (57.14% and 42.8% in group A and group B, respectively). The mean initial volume of ulcers in both groups was comparable. The mean number of dressings required in each group for re-epithelization was 3.5 in group A and 3.2 in group B. The mean percentage reduction seen between each dressing during the PRF procedure ranged from 44.8% to 71.4% and during the PRP procedure ranged from 46.1% to 73.8% [Figure 1]. The mean VAS for patient’s assessment of improvement was 9.2 ± 1.6 in group A and 8.9 ± 0.9 in group B.
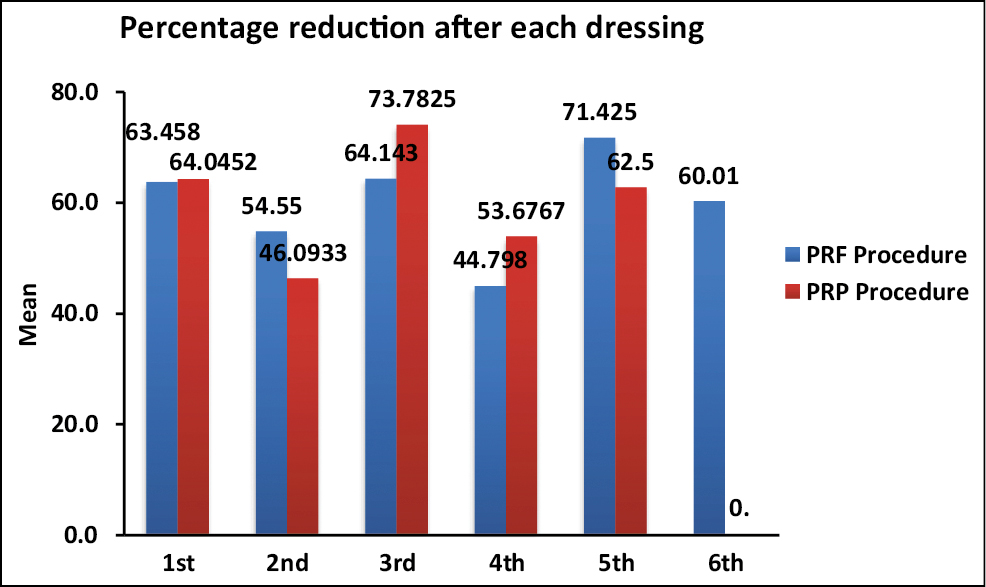
- Mean percentage reduction after each dressing between study procedures
Infections were seen in one ulcer in group A (4.8%) and two ulcers in group B (9.5%). Procedures had to be abandoned for two ulcers, one ulcer each from group A and group B because of the development of infection in ulcers. One ulcer patient from group B presented with infection at the two-week follow-up, after a complete re-epithelization. Patients with ulcers that developed infection gave complaints of pain and discharge from the ulcer. This was seen in one ulcer in the PRF group (4.8%) and two ulcers in the PRP group (9.5%). Recurrences were seen in four ulcers in the PRF group (19%) and three ulcers in the PRP group (14.3%). However, these recurrences were seen outside the duration of the study. Other ulcers showed no evidence of recurrence in either of the group during the duration of the study.
DISCUSSION
Chronic cutaneous ulcers have an impact on almost every aspect of a person’s day-to-day life. Chronic ulcers of the leg are a common cause of morbidity, and their prevalence in the community ranges from 1.9% to 13.1%.[3] Common aspects include the persistence of pain (exception—trophic ulcers caused by Hansen’s disease), friable granulation tissue, foul odor, and wound breakdown instead of healing, sleep disturbances, restriction of mobility, and work capacity, among other complaints. Social activities may be restricted because of the fear of injury and stigma from society. Thus, chronic nonhealing ulcers are associated with significant morbidity, loss of productivity, and reduced quality of life along with the high cost of healthcare.
Because of their essential role in homeostasis, platelets are deployed to sites of injury or infection to modulate inflammatory processes through the secretion of growth factors, chemokines, and other inflammatory mediators.[2] The majority of the secreted substances found in platelets are localized within granules.[3] Numerous growth factors with healing roles are released by activated platelets including insulin-like growth factor, epidermal growth factor, vascular endothelial growth factor, transforming growth factor-b1, and platelet-derived growth factors.[10] Platelet-rich preparations are a safe, reliable, and cost-effective means to accelerate healing and improve the probability of efficient repair following injury.[4]
A total of 42 ulcer cases were effectively evaluated in our study. The choice of treatment of ulcers was independent of morphological features, site, and severity. Our study showed a predominance of female patients, which was in contrast to the observation by Anandan et al. where there was male predominance. The mean age in the PRF group of our study was 46.2 ± 20.8 years, which was comparable to that in the study conducted by Nagaraju et al.[7] and in the PRP group of our study was 48.7 ± 19.1 years, which was similar to that seen in the study conducted by Anandan et al.[9] [Table 2].
| Characteristics | Our study, PRF versus PRP | Anandan et al.,9 PRP | Nagaraju et al.,7 PRF | Frykberg et al.,11 PRP | Suryanarayan et al.,12 PRP | Shreyas et al.,2 PRF vs conventional therapy | Suthar et al.,4 PRP |
|---|---|---|---|---|---|---|---|
| Gender | Female predominance | Male predominance | Male predominance | - | - | Male predominance | Male predominance |
| Age (years) | PRF: 46.2 ± 20.8 PRP: 48.7 ± 19.1 |
41.9 | 38.33 | 60.6 ± 14.7 | 42.5 | PRF: 35.16 ± 15.824 Conventional: 41.08 ± 16.731 |
62.5 ± 13.53 |
| Site | Lower limb—right foot | Lower limb—foot | Lower limbs | Lower limb | Lower limb | Lower limbs | |
| Most common cause of nonhealing ulcer | Hansen’s disease | Only Hansen’s disease considered | Only Hansen’s disease considered | Pressure ulcers | Venous | Traumatic | Venous |
| Mean healing time (weeks) | PRF: 3.5 PRP: 3.2 |
4.38 | 3 | 2.8 ± 2.4 | 5.6 ± 3.23 | PRF: 3.52 Conventional: 4.19 |
8.2 ± 1.9 |
| Mean percentage reduction in area/volume of ulcer | PRF: 10.8–71.4 PRP: 50.9–63 |
Re-epithelization: 92% | Area improvement: 93.5% (first) 97.74% (second) |
Area: 39.5% ± 41.2% Volume: 51% ± 43.1% |
Area: 91.7% ± 18.4% Volume: 95% ± 14% |
Surface area improvement seen in the PRF group | Wound size reduction >90% in 70.83% |
| Maximum number of dressings required | 6 | 6 | 5 | - | 6 | 8 | 4 |
Of the total number of ulcers in our study, 15 ulcers (71.4%) in group A (PRF) and 16 ulcers (76.2%) in group B (PRP) were trophic ulcers in patients with Hansen’s disease, whereas six ulcers (28.6%) in the PRF group and five ulcers (23.8%) in the PRP group were caused by other dermatological complaints like infected eczema, systemic sclerosis, keloid, and necrolytic acral erythema. Lower limbs were the most common site of ulcers seen in our study in both groups. This was similar to the sites seen in studies conducted by Anandan et al.[9]
A uniform reduction in the volume of ulcers was seen per dressing in both procedures. Both groups showed similar efficacy in achieving re-epithelization as the mean number of dressings required in each group were comparable [Figures 2,3,4-5]. The observations were similar to the study conducted by Anandan et al., where the mean time for ulcer healing with PRP was 4.38 weeks.[9] Furthermore, a study conducted by Frykberg et al.[11] showed a mean healing time of 2.8 weeks and that by Suryanarayan et al.[12] showed a mean duration of healing of 5.6 ± 3.23 weeks. The above-mentioned studies were done in chronic leg ulcers due to various causes, with only a few patients with trophic ulcers secondary to Hansen’s disease.
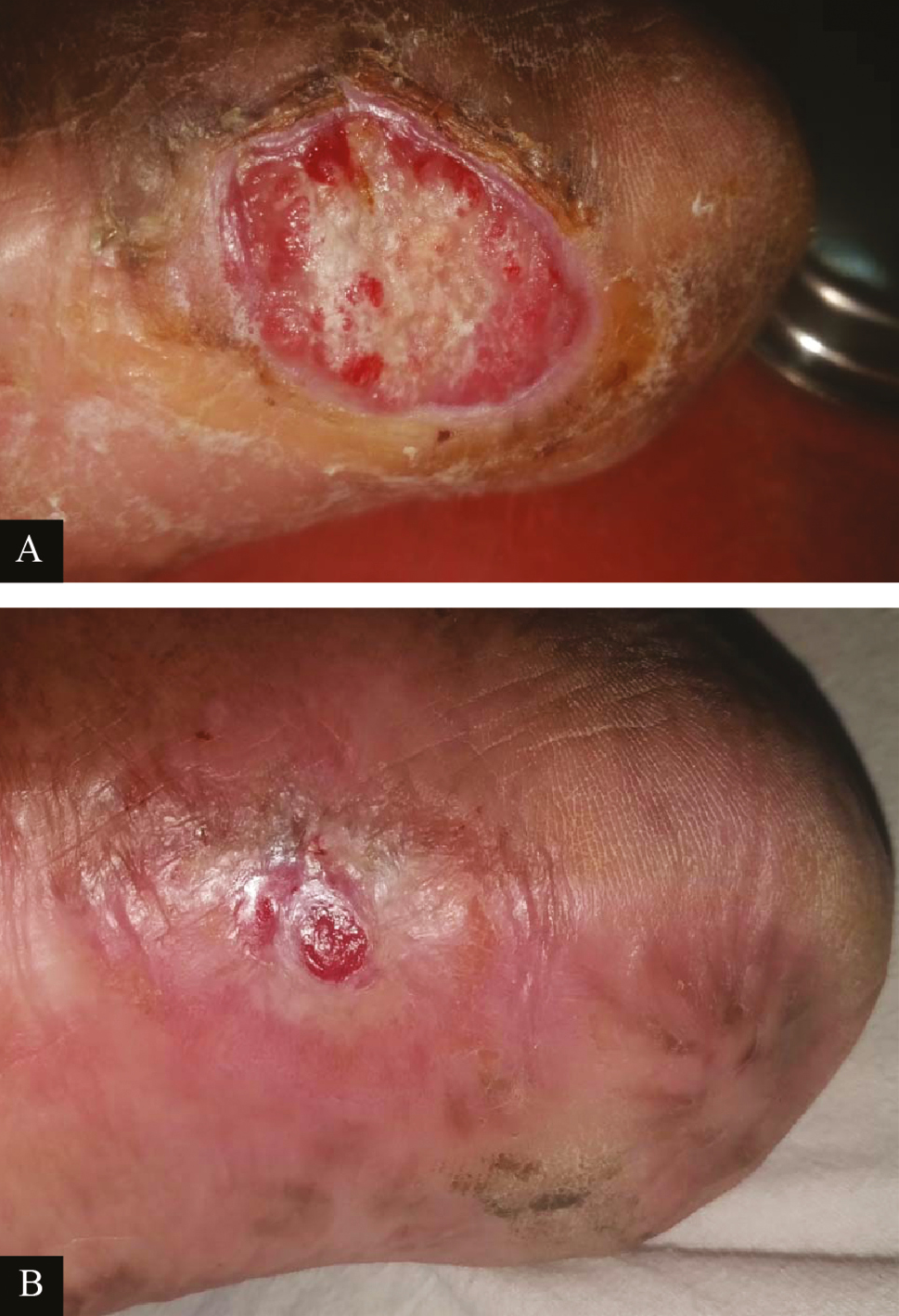
- Clinical picture of platelet-rich fibrin dressings in ulcer due to Hansen’s disease: (A) at baseline (B) after sixth platelet-rich fibrin dressing (re-epithelized)
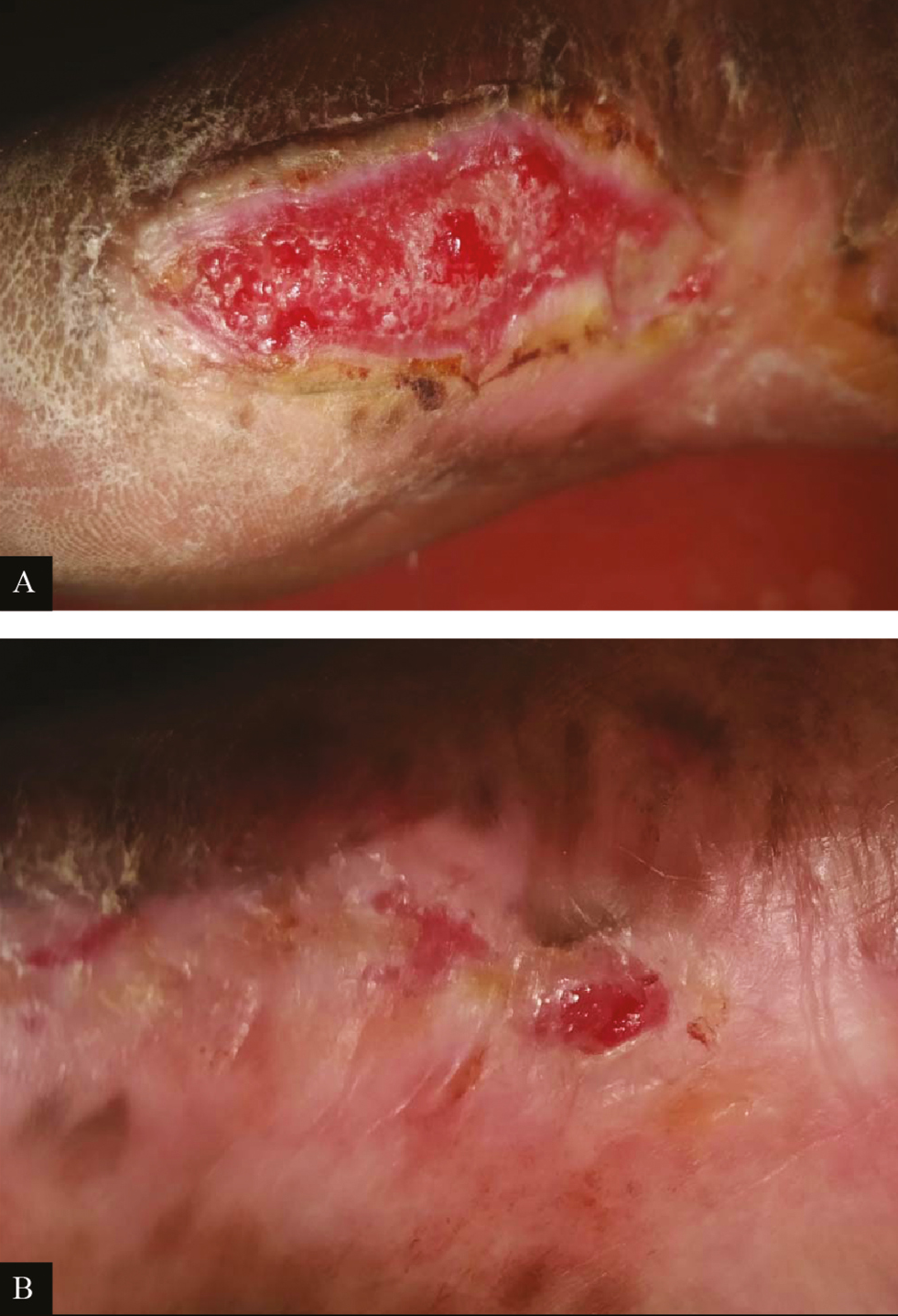
- Clinical picture of PRP dressing in ulcer due to Hansen’s disease: (A) at baseline (B) after fourth PRP dressing
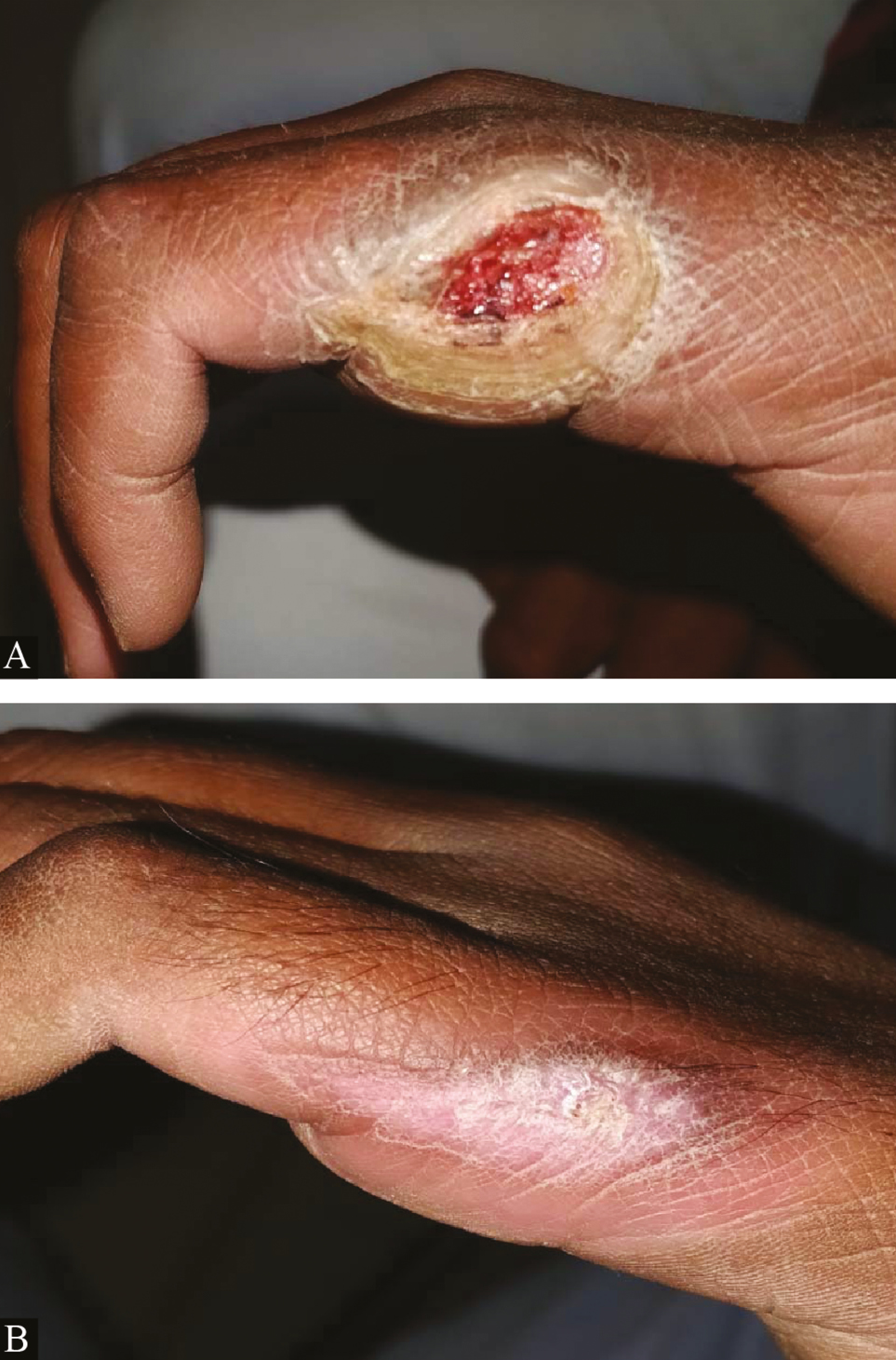
- Clinical picture of platelet-rich fibrin dressings in ulcer due to Hansen’s disease: (A) at baseline (B) after third platelet-rich fibrin dressing (re-epithelized)
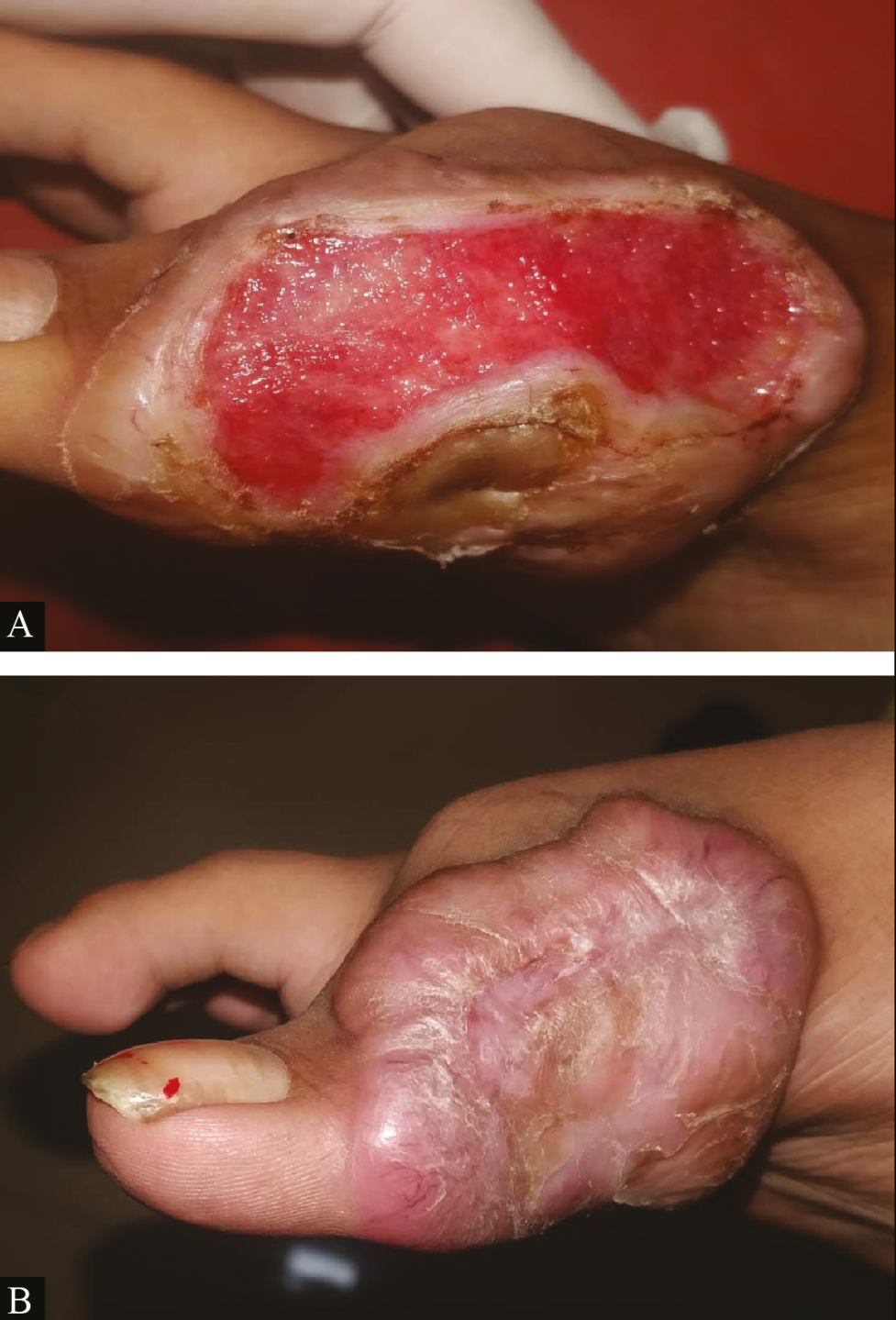
- Clinical picture of platelet-rich fibrin dressing in ulcer due to keloid with chronic ulcer: (A) at baseline (B) after fourth platelet-rich fibrin dressing
The response to treatment in our study was assessed by the percentage reduction in the volume of ulcers per dressing. In both groups, there was uniformity in percentage reduction of the volume of ulcers per dressing per week, with no significant difference in the percentage reduction in any one particular procedure. This was similar to the study conducted by Nagaraju et al., which showed a mean percentage improvement in the volume of 97.74% at the end of the second sitting using platelet rich fibrin membrane with a maximum requirement of five sittings for all ulcers.[7]
The study also noted that the ulceration found in Hansen’s disease is a result of nerve damage and cutaneous anesthesia and not as a consequence of the infection itself. Other factors causing a delay in healing of ulcers in Hansen’s disease include a decrease in vascularization, growth factors, and nutrition.[2] Similarly, in our study, there was no correlation between the presence of chronic ulcers and the activity of the disease. The mean percentage reduction of volume of ulcers caused by Hansen’s disease in group A as seen in Figures 2 and 4, which underwent PRF dressings, was in the range of 10.8%–71.4%, and 55.2%–78.8% in ulcers caused by other dermatological conditions as seen in Figure 5. In group B, i.e., chronic ulcers undergoing PRP dressings as seen in Figure 3 showed the mean percentage reduction of ulcer volume in the range of 50.9%–63% in ulcers caused by Hansen’s disease and 22%–87.1% in ulcers caused by non-Hansen’s conditions. This was also true when comparing the mean number of dressings required for ulcers caused by Hansen’s disease (3.3) and those caused by non-Hansen’s complaints (3.6).
The study conducted by Shreyas et al., where efficacy of PRF therapy was compared with conventional dressing, showed significant differences in both the subjective surface area and the objective surface area improvement with better healing in the PRF group.[2] The fibrin network in PRF has a homogeneous, three-dimensional organization, even more highly coherent than natural fibrin clots. These preliminary data, therefore, imply that PRF would not only be a new generation of platelet gel, but a completely usable healing concentrate.[213,14] The leukocytes and platelet-rich patch embedded in the fibrin clot can thus provide a way of transferring concentrated cells and signals directly to a surface and would be beneficial for the healing of recalcitrant wounds.
Shreyas et al. further observed that, unlike PRP, PRF does not dissolve quickly after application and hence provides a strong fibrin matrix that is slowly remodeled in a way similar to that of a blood clot.[214] The study also found that PRF was helpful in volume-deficient wounds, where the PRF applied transformed into corresponding adjacent tissue muscle, subcutis, and skin, thus showing that weekly PRF dressing showed better healing as compared to conventional dressing. However, this study had limitations in comparing efficacy of PRP versus PRF.
Our study was able to quantify the percentage reduction in the volume of ulcers at each sitting in both groups and hence was able to overcome this limitation. In our study, we found that percentage reduction in the volume of ulcers in both groups was comparable, and both procedures were similar in their efficacy toward the healing of the ulcer.
The effectiveness of both procedures was seen with complete re-epithelization in 20 ulcer cases in group A (95.2%) and 19 ulcer cases in group B (90.4%). Our study also showed that both PRF and PRP had a good capacity in the regeneration of tissues in chronic cutaneous ulcers, irrespective of etiology. Subjective assessment by patients on the basis of VAS furthermore showed a high mean VAS at the end of the treatment period in both groups. This further proves that both forms of procedures were well received by patients and has a high rate of patient satisfaction for healing of chronic cutaneous ulcers.
Studies conducted by Suthar et al. established safety and efficacy along with improvement in the quality of life in a patient undergoing weekly PRP dressings.[4] Studies conducted by Dohan et al.,[14] Suchetha et al.,[15] and Yazawa et al.[16] concluded that although PRP had a higher platelet concentration when compared with PRF, superior effects were seen in the use of PRF when compared with PRP as when the growth factors were incorporated into drug delivery systems such as fibrin, the mean concentration of growth factors in the platelet concentrates was three times or more than that observed with conventional PRP. Also, in PRF, the growth factors were released in a controlled manner over approximately one week. Thus, slower, controlled, and consistent release of growth factors from platelet rich fibrin membrane than PRP, hence, showed better healing properties with PRF.[141516] In contrast, in our study, we observed that the mean number of dressings and mean percentage reduction in the volume of ulcers in both groups were comparable with no statistically significant difference in outcomes of ulcers in both groups.
Both modalities of therapy show low rates of complications and recurrences. Infection along with serous/seropurulent discharge was the most common complication seen in both groups. This can be reduced by appropriate antibiotic therapy prior to the procedure, based on culture and sensitivity, and following adequate aseptic precautions during the procedure. Patients also should be educated regarding the care of ulcers and dressing during ongoing treatment, especially if the procedure is carried out on an outpatient basis. Patients should also be advised regarding limb care and the use of appropriate footwear, especially in cases of Hansen’s disease, where sensations in the limbs are greatly diminished or absent on account of the disease itself, and the neglect of the limb may lead to recurrences or formation of new ulcers.
CONCLUSION
Our study aimed at assessing the efficacy of PRF versus PRP as a regenerative strategy for chronic cutaneous ulcers. Factors including etiology, nature, and duration of ulcer, and demographic distribution were uniform across both study groups. The cost of the procedure was economical and showed good patient compliance. Both procedures can also be done on an outpatient as well as inpatient basis. Our study was able to show significant improvement in the reduction of the volume of ulcers after every follow-up in both groups.
Both procedures gave similar results and were highly efficacious with minimal complications and well received by patients undergoing treatment, as assessed by percentage reduction of the volume of ulcers, re-epithelization, and VAS. Thus, PRF and PRP dressings provide a safe, effective, and inexpensive modality of therapy and hence can be used as a regenerative medicine strategy in the healing of chronic cutaneous ulcers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Authors’ contribution
Vartika R. Ratan contributed to data collection, analysis and interpretation, and drafting of the article. Arun C. Inamadar contributed to critical revision of article and final approval of the version to be published.
Informed consent
Written and informed consent was taken.
REFERENCES
- Wound healing. In: Brunicardi F, Andersen D, Billiar T, Dunn D, Hunter J, Matthews J, eds. Schwartz’s Principles of Surgery (10th ed.). NewYork: McGraw-Hill; 2014. p. :259-61.
- [Google Scholar]
- Efficacy of autologous platelet-rich fibrin in chronic cutaneous ulcer: A randomized controlled trial. IP Indian J Clin Exp Dermatol. 2017;3:172-81.
- [Google Scholar]
- Chronic leg ulcers: Epidemiology, aetiopathogenesis, and management. Ulcers. 2013;2013:9.
- [Google Scholar]
- Treatment of chronic non-healing ulcers using autologous platelet rich plasma: A case series. J Biomed Sci. 2017;24:16.
- [Google Scholar]
- Platelet-rich preparations to improve healing. Part I: Workable options for every size practice. J Oral Implantol. 2014;40:500-10.
- [Google Scholar]
- Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991;45:319-26.
- [Google Scholar]
- Autologous platelet-rich fibrin matrix in non-healing trophic ulcers in patients with Hansen’s disease. J Cutan Aesthet Surg. 2017;10:3-7.
- [Google Scholar]
- A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52:68-70, 72, 74 passim.
- [Google Scholar]
- Platelet rich plasma: Efficacy in treating trophic ulcers in leprosy. J Clin Diagn Res. 2016;10:WC06-9.
- [Google Scholar]
- Chronic wound healing: A review of current management and treatments. Adv Ther. 2017;34:599-610.
- [Google Scholar]
- Chronic wounds treated with a physiologically relevant concentration of platelet-rich plasma gel: A prospective case series. Ostomy Wound Manage. 2010;56:36-44.
- [Google Scholar]
- Efficacy of autologous platelet-rich plasma in the treatment of chronic nonhealing leg ulcers. Plast Aesthet Res. 2014;1:65-9.
- [Google Scholar]
- Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30:145-51.
- [Google Scholar]
- Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37-44.
- [Google Scholar]
- Platelet concentration in platelet concentrates and periodontal regeneration—Unscrambling the ambiguity. Contemp Clin Dent. 2015;6:510-6.
- [Google Scholar]
- Basic studies on the clinical applications of platelet-rich plasma. Cell Transplant. 2003;12:509-18.
- [Google Scholar]






