Translate this page into:
Serum interleukin-6 and high sensitivity C-reactive protein levels and their correlation with the vitiligo disease activity and extent: A cross-sectional study of 58 patients
*Corresponding author: Keshavmurthy A. Adya, Department of Dermatology, Venereology and Leprosy, Shri B M Patil Medical College, Hospital and Research Center, BLDE (Deemed to be University), Vijayapur, Karnataka, India. adyamurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Uttmani BM, Adya KA, Inamadar AC. Serum interleukin-6 and high sensitivity c-reactive protein levels and their correlation with the vitiligo disease activity and extent: A cross-sectional study of 58 patients. J Cutan Aesthet Surg. 2024;17:266-71. doi: 10.4103/JCAS.JCAS_12_23
Abstract
Vitiligo is an acquired depigmenting disorder due to the destructive loss of melanocytes, clinically presenting as hypopigmented or depigmented macules and/or patches. Many theories have been proposed to explain its etiopathogenesis among which cell-mediated immunity is one of the crucial links. Estimation of vitiligo activity and extent in a patient is important in tailoring an optimal treatment regimen. Interleukin-6 (IL-6) and high-sensitivity C-reactive protein (HsCRP) are sensitive indicators for systemic inflammation and are found to be relevant in determining vitiligo disease activity. This study was conducted to estimate serum levels of IL-6 and HsCRP in vitiligo patients and to correlate them with the disease activity and extent in order to assess if these serum markers serve as objective indicators of vitiligo disease activity. This was a hospital-based cross-sectional study of 58 vitiligo patients diagnosed clinically irrespective of age, gender, and any ongoing or past treatment. Disease activity and extent were calculated using the vitiligo disease activity (VIDA) score and vitiligo area severity index (VASI), respectively. Serum levels of IL-6 and HsCRP were obtained and their correlation with VIDA and VASI values were statistically analyzed. A weak negative statistically insignificant correlation was found between IL-6 and VIDA (P = 0.092). No correlation was found between VIDA and HsCRP (P = 0.998). A weak positive, statistically insignificant correlation was found between VASI and IL-6 as well as between VASI and HsCRP (P = 0.175 and P = 0.238, respectively). Although statistically insignificant, the patients who were not on immunosuppressive therapy showed higher mean values of IL-6 and HsCRP compared to those who were on immunosuppressive therapy. In contrast to the findings of previous studies, our study found a weak negative correlation between VIDA and IL-6 levels possibly attributable to the difference between the mean levels of IL-6 among the subgroups of patients who were, and were not on immunosuppressive therapy. The VIDA score and HsCRP levels did not show any statistical correlation. However, patients who were not on immunosuppressive therapy showed a higher albeit statistically insignificant mean value of HsCRP. Our observations suggest that any ongoing and/or treatment in the recent past, especially immunosuppressive therapy, and any co-morbidities should be essentially considered while investigating for sensitive serum markers of inflammation as determinants of vitiligo disease activity.
Keywords
HsCRP
IL-6
VASI
VIDA
Vitiligo
INTRODUCTION
Vitiligo is an autoimmune disorder characterized by hypopigmented and/or depigmented macules and/or patches due to the selective destruction of melanocytes. It has a worldwide prevalence of 0.5%–2%.1 Prevalence in India ranges from 0.25% to 4%.2 Age of onset of vitiligo has a wide range and shows two summits in some studies. Vitiligo generally begins by the age of 30 years and several reports indicate that in half of the patients it begins by 20 years of age.1,3
The exact etiology of vitiligo is unclear and various theories regarding its etiopathogenesis have been postulated. Cell-mediated immunity appears to be one of the key links in driving the disease.1 Interleukin-6 (IL-6) is a T-helper 2 cytokine that has a role in immune regulation and inflammation and has been associated with numerous systemic inflammatory diseases.4 An emergence of epidermal cytokine imbalance in the pathogenesis of vitiligo has now been suggested by changes in the levels of keratinocyte-derived mediators in the epidermis of vitiligo-affected skin.4-6 C-reactive protein (CRP) is another sensitive marker of inflammation. Tests for high- sensitivity CRP (HsCRP) are capable of detecting very low levels of CRP in blood and have been utilized as a diagnostic and prognostic indicator of cardiovascular diseases.7,8
Estimation of vitiligo disease activity and extent is essential in formulating an optimal treatment regimen for the patients. The vitiligo disease activity (VIDA) score is frequently utilized to clinically determine the disease activity.9,10 Vitiligo area severity index (VASI) is used to clinically calculate the extent of disease.10,11 These methods however are subjective as VIDA relies upon the patients’ ability to recall the development of new lesions and/or extension of the preexisting lesions. The VASI may show inter-observer variations as it is calculated manually. Clinical features such as the Koebner phenomenon, confetti-like depigmentation, hypopigmented areas, and blurred edges indicate disease activity. However, they may not be seen in all patients.7 Hence, there is a need for objective ways to determine the vitiligo disease activity. Epidermal spongiosis, epidermal and dermal lymphocytes, basal cell vacuolization, and dermal melanophages are histological indicators of disease activity in the lesional skin.12 The inclusion of immunohistochemistry (such as CD4 and CD8 staining) further adds essence.13 However, such evaluation requires an invasive skin biopsy that is not routinely performed in vitiligo. With the growing and promising evidence of serum biomarkers as determinants of vitiligo disease activity, their estimation offers a simple and objective method to determine vitiligo disease activity. Estimation of serum IL-6 and HsCRP levels may be helpful in this regard for the reasons described above.
MATERIALS AND METHODS
This was a hospital-based cross-sectional study involving 58 clinically diagnosed vitiligo patients attending the authors’ department between January 2021 and May 2022. All the patients presenting with vitiligo irrespective of age, gender, and ongoing or past treatment were enrolled. Patients with co-existing chronic inflammatory disorders such as rheumatoid arthritis, alopecia areata, psoriasis, etc., active cutaneous or systemic infections, and co-morbidities such as hypertension, diabetes mellitus, and ischemic heart disease were excluded. After obtaining informed written consent, details of the disease in regard to onset, duration, evidence of activity, and other relevant information were recorded. Each patient’s disease was categorized into one of the clinical types (vitiligo vulgaris, segmental, acral, acrofacial, and focal) based on the morphology and distribution of the lesions. The disease activity and extent were determined by VIDA and VASI scoring [Table 1]. Serum levels of IL-6 and HsCRP were obtained and their values were statistically correlated with the VIDA and VASI scores.
| The VIDA is a 6-point scoring based on the patients’ recall of appearance of new lesions or expansion of existing lesions as measures of disease activity, and no change in existing lesions and appearance of no new lesions as a measure of stability. The scoring is done as below | The VASI refers to the calculation of body surface area affected by vitiligo in terms of hand units, where one hand unit (palm+volar aspect of all fingers) approximates to 1% of the total body surface area. The scoring is done as below | ||
|---|---|---|---|
| Disease Activity | Score | Degree of pigmentation | Percentage of BSA involved |
| Activity of 6 weeks or less | 4+ | Complete depigmentation | 100 |
| Activity of 6 weeks to 3 months | 3+ | Specks of pigmentation | 90 |
| Activity of 3–6 months | 2+ | Depigmented area exceeds the pigmented area | 75 |
| Activity of 6–12 months | 1+ | Pigmented and depigmented areas equal | 50 |
| Stable for 1 year or more | 0 | Pigmented area exceeds depigmented areas | 25 |
| Stable with spontaneous repigmentation | |||
| for 1 year or more | −1 | Only specks of depigmentation | 10 |
VIDA: Vitiligo disease activity, VASI: Vitiligo area severity index, BSA: Body surface area
Statistical analysis of the data collected was performed using the Statistical Package for the Social Sciences (IBM Corp. 2011. IBM SPSS Statistics for Windows, Version 20.0, Armonk, New York). Results were presented as mean (median) ± standard deviation, counts, percentages, and diagrams. Categorical variables were compared using the chi-square test. The correlation between variables was calculated by Person’s/Spearman’s correlation. A value of P < 0.05 was considered statistically significant. All statistical tests performed were two-tailed.
RESULTS
Patient and disease characteristics
Amongst the 58 study entrants, 34 (58.6%) were females and 24 (41.4%) were males, with a female-to-male ratio of 1.41:1. The age group of patients ranged from 4 to 65 years (mean 30.43 years) and most of them (13 [22.4%]) belonged to the second decade. Disease duration ranged from 0.5 to 240 months with a mean of 34.258 months. Most of the patients (14 [24.1%]) had the disease for a duration between 18 and 24 months. The most common type of vitiligo was vitiligo vulgaris in 42 (72.4%) patients followed by segmental in 7 (12%). The second decade was the age group exhibiting the most number of vitiligo vulgaris cases (11 [26.2%]), while segmental vitiligo was exclusive to the 5 to 16 years age group (7 [12%]). Thirty-five (60.3%) patients among the total were on immunosuppressive treatment.
Relation between patient demographics (age and gender) and the indices of disease activity (VIDA) and extent (VASI)
Most of the study entrants (27 [46.55%]) showed a VIDA score of 4+, followed by 11 (18.9%) patients showing a score of 2+. Stable disease as per VIDA score (of 0) was noted in 9 (15.5%) patients. The highest proportion of the cases exhibiting a VIDA score of 4+ belonged to the age group of <10 years (6/9 [66.6%]), while the age group showing the highest proportion of stable disease was 60-69 years (1/3 [33.3%]) [Table 2]. Among the 27 patients showing a VIDA score of 4+, 10 were males and 17 females, with a male-to-female ratio of 1:1.7.
| Age groups (in years) | VIDA | Chi-square value | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | |||
| <10 (n=9) | 1 | 0 | 0 | 2 | 6 | 24.887 | 0.412 |
| 10-19 (n=8) | 2 | 0 | 2 | 1 | 3 | ||
| 20-29 (n=13) | 3 | 1 | 1 | 2 | 6 | ||
| 30-39 (n=8) | 1 | 1 | 4 | 0 | 2 | ||
| 40-49 (n=6) | 0 | 0 | 1 | 2 | 3 | ||
| 50-59 (n=11) | 1 | 0 | 1 | 2 | 7 | ||
| 60-69 (n=3) | 1 | 0 | 2 | 0 | 0 | ||
| Total (n=58) | 9 | 2 | 11 | 9 | 27 | ||
VIDA: Vitiligo disease activity
As to VASI, the majority (39 [67.2%]) belonged to the 10% group followed by 18 (31.03%) patients in the 25% group. Only one (1.7%) patient had a VASI score of 50%. The highest proportion of cases exhibiting a 10% VASI score were from the second decade (12/13 [92.3%]). Among the 39 patients belonging to the 10% VASI group, 18 were males and 21 females, with a male-to-female ratio of 1:1.16.
Relation between the indices of disease activity (VIDA) and extent (VASI) and the serum markers (IL-6 and HsCRP)
Mean serum levels of IL-6 and HsCRP noted were 9.3 pg/mL and 3.08 mg/L, respectively. A weak negative, statistically insignificant correlation was found between VIDA and IL-6 levels [Table 3 and Figure 1a]. No statistical correlation was found between VIDA and HsCRP levels [Table 3 and Figure 1b]. A weak, statistically insignificant correlation between VASI and both the markers was observed [Table 3, and Figure 2a and b].
| Variables | Spearman’s rho correlation coefficient | P–value |
|---|---|---|
| VIDA and IL-6 | -0.223 | 0.092 |
| VIDA and HsCRP | 0 | 0.998 |
| VASI and IL-6 | 0.180 | 0.175 |
| VASI and HsCRP | 0.157 | 0.238 |
Correlation is significant at the 0.01 level (2-tailed), VIDA: Vitiligo disease activity, VASI: Vitiligo area severity index, HsCRP: high-sensitivity C-reactive protein, IL-6: Interleukin-6
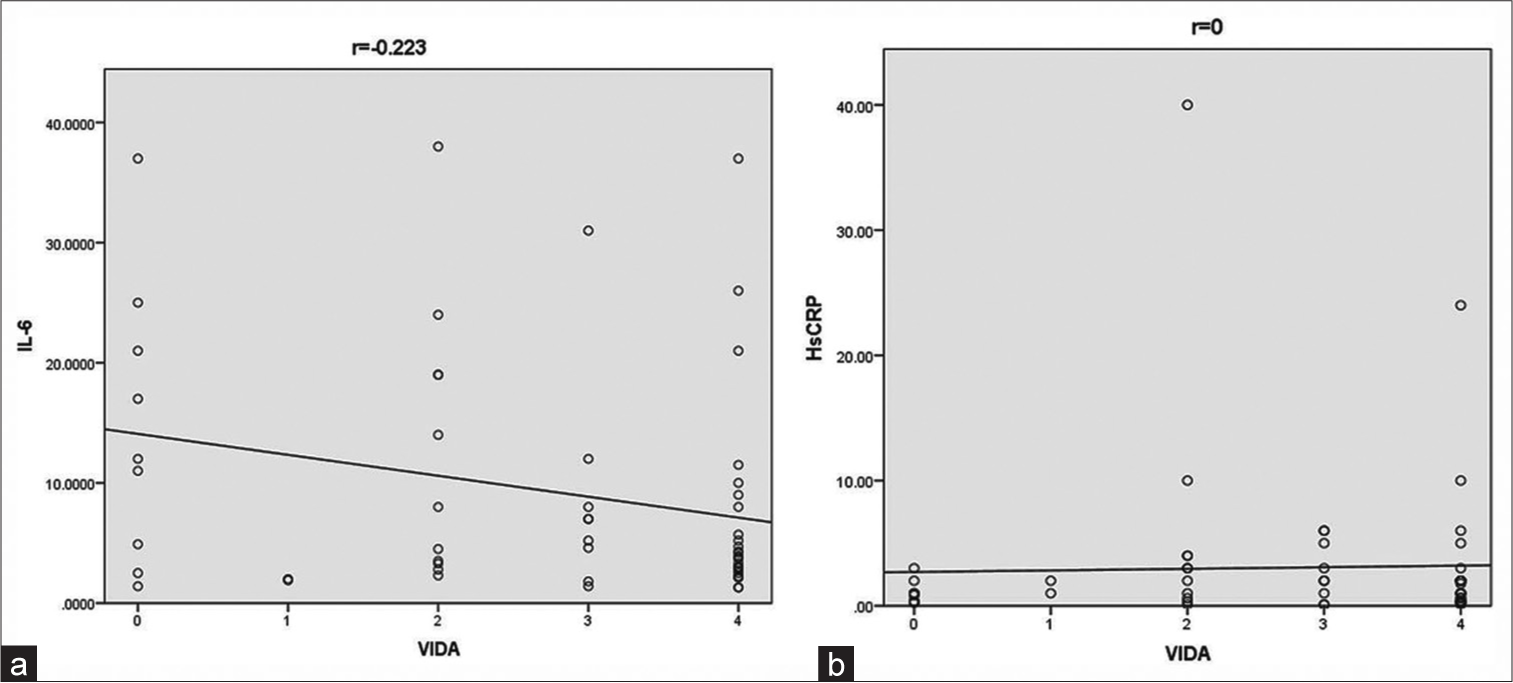
- (a) Scatter diagram showing correlation between vitiligo disease activity and interleukin-6 and (b) between vitiligo disease activity and high sensitivity C-reactive protein. HsCRP: High-sensitivity C-reactive protein, IL-6: Interleukin-6, VIDA: Vitiligo disease activity.
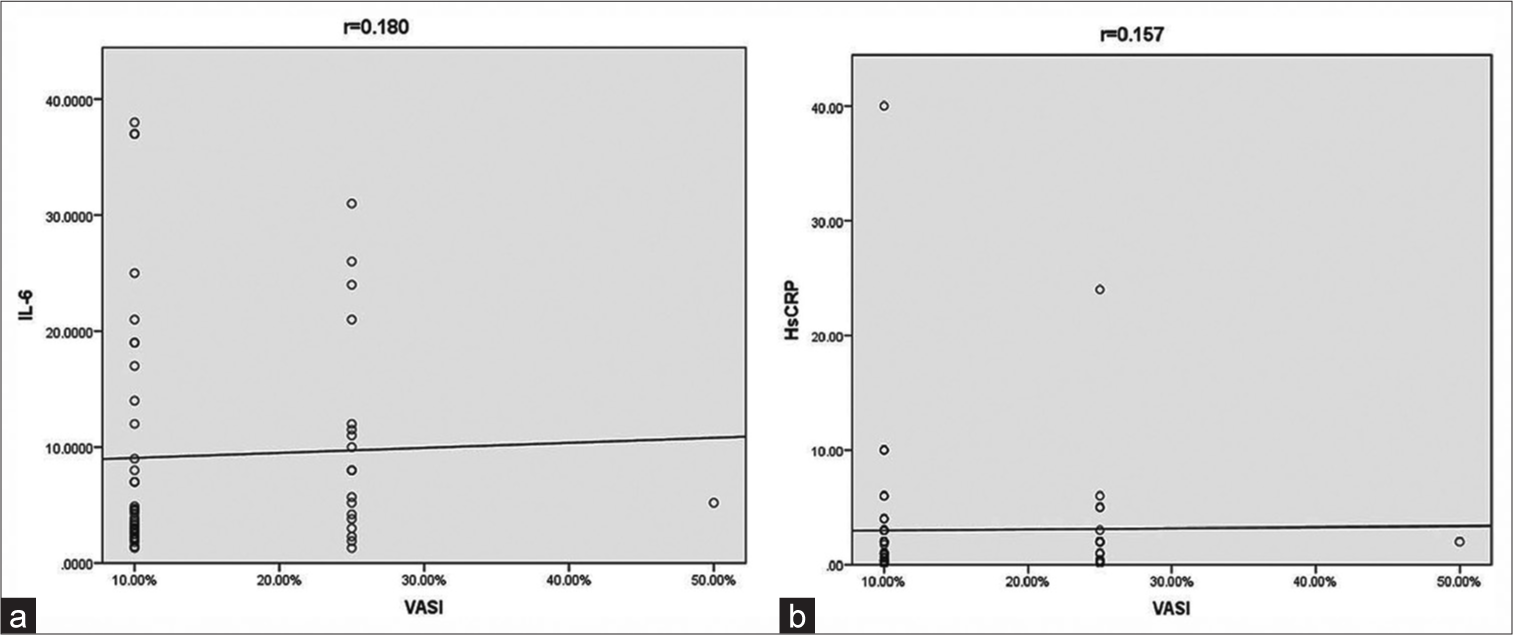
- (a) Scatter diagram showing correlation between vitiligo area severity index and interleukin-6 and (b) between vitiligo area severity index and high sensitivity C-reactive protein. HsCRP: High-sensitivity C-reactive protein, IL-6: Interleukin-6, VASI: Vitiligo area severity index.
Relation between the levels of serum markers (IL-6 and HsCRP) and treatment
The mean values of both IL-6 and HsCRP levels were higher in the 23 (39.6%) patients who were not receiving any immunosuppressive treatment compared to those (35 [60.3%]) who were on immunosuppressants [Table 4 and Figure 3]. However, the difference was statistically insignificant.
| Variables | Group | N | Mean | SD | Mann-Whitney U test value | P-value |
|---|---|---|---|---|---|---|
| IL-6 | NOT* | 35 | 11.351 | 10.889 | 505 | 0.105 |
| ON# | 23 | 6.165 | 6.885 | |||
| HsCRP | NOT* | 35 | 3.66 | 7.586 | 397.5 | 0.943 |
| Non Significant | ON# | 23 | 2.207 | 2.369 |
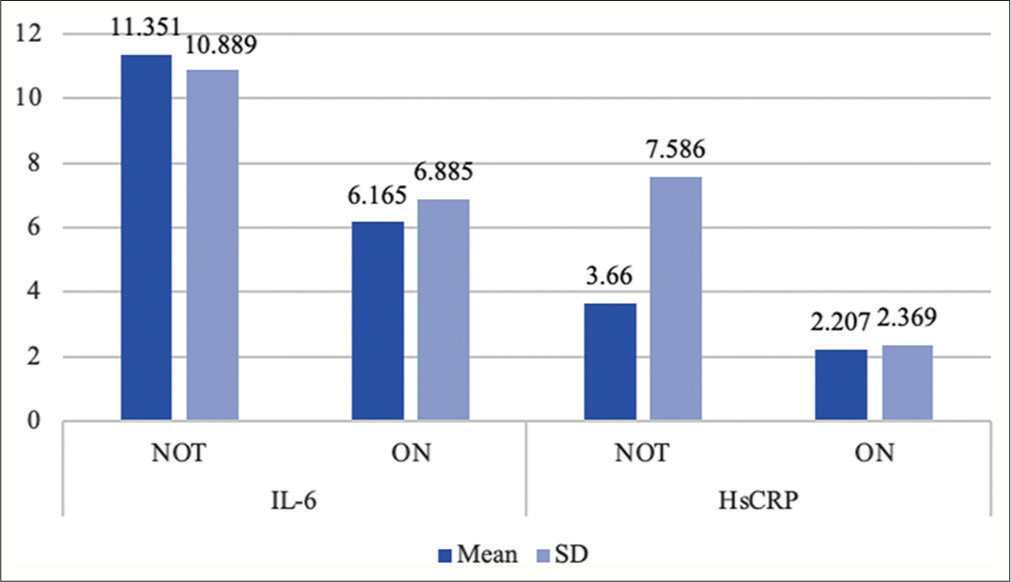
- Distribution of mean values and standard deviation of interleukin-6 and high sensitivity C-reactive protein levels in subgroups of patients on and not on immunosuppressive therapy. HsCRP: High-sensitivity C-reactive protein, IL-6: Interleukin-6, SD: Standard deviation.
DISCUSSION
In line with previous studies, majority of our study entrants were females, and majority belonged to the second decade.8,14,15 Similar to the previous observations, children accounted for the age group exhibiting high disease activity the most, attributable to the frequent autoimmune nature of the disease in them.16,17
Compared to healthy controls, Singh et al.18 and Habib et al.19 noted higher levels of IL-6 in vitiligo patients which however did not significantly correlate with VIDA scores. We noted a weak negative, statistically insignificant correlation between IL-6 and VIDA scores. Our observation could be attributable to the non-exclusion of patients with ongoing immunosuppressive treatment and the low levels in them influenced the overall correlation between serum IL-6 levels and VIDA. A weak positive, statistically insignificant correlation between VASI and IL-6 was however observed in our study.
Sarkar et al.20 and Rahman et al.21 found a statistically significant difference in HsCRP levels between the vitiligo case and control groups along with a positive correlation between HsCRP and VASI. In contrast, we noted no correlation between VIDA and HsCRP similar to the observations of Namazi et al.8 This is also attributable to the non-exclusion of patients with ongoing immunosuppressive treatment described above. However, a weak positive, statistically insignificant correlation between VASI and HsCRP was found.
Segmental vitiligo may be considered distinctive as it differs from the non-segmental disease in having an early onset, exhibiting an early phase of rapid progression followed by dormancy, having frequent association with leucotrichia, and showing relative refractoriness to conventional treatment modalities. As to its pathogenesis, an inflammatory response (cytotoxic CD8 T-cell mediated) has been demonstrated by several studies. However, autoimmunity needs to be explored in detail as the existing data show the less frequent association of segmental than non-segmental vitiligo with systemic autoimmune diseases.22 Among our study entrants, 7 had segmental vitiligo and all of them were constituted by children. Majority of them (4/7 patients) showed a VIDA score of 4+ indicating higher disease activity. Most of them (6/7 patients) however belonged to the 10% VASI group. Three of these 7 patients had elevated IL-6 levels, 1 showed elevation in both IL-6 and HsCRP levels, and the rest showed normal levels of IL-6 and HsCRP. The patient with elevation in both the markers also had a more widespread disease (VASI 25%) and higher activity (VIDA 4+). None of the patients with elevated markers were on immunosuppressive therapy. These observations support the disease characteristics mentioned above.
Limitations
The main limitation of our study was the lack of a healthy control group. As the study intended to evaluate the disease activity in all patients presenting to us, we excluded only the patients with concurrent inflammatory, autoimmune, and disorders like diabetes, hypertension, and ischemic heart diseases (that could have led to erroneously high serum IL-6 and HsCRP levels), but not the patients with on-going treatment. This is another limitation influencing the study outcomes.
CONCLUSION
Serum markers of inflammation may serve as predictors of disease activity in vitiligo. However, their values may be influenced by several factors such as co-morbidities and any ongoing, or therapy in the recent past. Therefore, these factors should be essentially considered while investigating for sensitive serum markers of inflammation as determinants of vitiligo disease activity.
Authors’ Contributions
All the authors contributed to the research study. Bhargavi M. Uttmani: Concepts, Design, Definition of intellectual content, Literature search, Manuscript preparation, Manuscript Editing, and Manuscript review. Keshavmurthy A. Adya: Concepts, Design, Definition of intellectual content, Literature search, Manuscript preparation, Manuscript Editing, and Manuscript review. Arun C. Inamadar: Concepts, Design, Definition of intellectual content, Literature search, Manuscript preparation, Manuscript Editing, and Manuscript review.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Investigation of the role of interleukin 6 in vitiligo pathogenesis. Immunol Investig. 2022;51:120-37.
- [CrossRef] [PubMed] [Google Scholar]
- An update on vitiligo pathogenesis. Pigment Cell Melanoma Res. 2021;34:236-43.
- [CrossRef] [PubMed] [Google Scholar]
- New insights into the pathogenesis of vitiligo: Imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res. 2002;15:87-92.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of hypersensitive-C reactive protein in vitiligo. Indian Dermatol Online J. 2018;9:53-4.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing the dynamic changes in vitiligo: Reliability and validity of the vitiligo disease activity score (VDAS) and vitiligo disease improvement score (VDIS) J Eur Acad Dermatol Venereol. 2022;36:1334-41.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment methods for the evaluation of vitiligo. J Eur Acad Dermatol Venereol. 2012;26:1463-71.
- [CrossRef] [PubMed] [Google Scholar]
- Disease severity indexes and treatment evaluation criteria in vitiligo. Dermatol Res Pract. 2011;2011:750342.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathology and molecular pathology of vitiligo [Internet] Depigmentation. IntechOpen 2019 Available from: http://dx.doi.org/10.5772/intechopen.84258
- [CrossRef] [Google Scholar]
- Biomarkers of disease activity in vitiligo: A systematic review. Autoimmun Rev. 2017;16:937-45.
- [CrossRef] [PubMed] [Google Scholar]
- CXCL-10 and Interleukin-6 are reliable serum markers for vitiligo activity: A multicenter cross-sectional study. Pigment Cell Melanoma Res. 2018;31:330-6.
- [CrossRef] [PubMed] [Google Scholar]
- The study of serum level of interleukin-2, interleukin-6, and tumor necrosis factor-alpha in stable and progressive vitiligo patients from sina hospital in tabriz, Iran. Indian J Dermatol. 2021;66:366-70.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-epidemiological profile of childhood vitiligo. Indian J Paediatr Dermatol. 2015;16:23.
- [CrossRef] [Google Scholar]
- Clinico epidemiological study of vitiligo in children. IP Indian J Clin Exp Dermatol. 2020;6:79-83.
- [CrossRef] [Google Scholar]
- Serum concentration of IL-6, IL-2, TNF-α and IFNγ in Vitiligo patients. Indian J Dermatol. 2012;57:12-4.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of serum total antioxidant status and interleukin-6 in vitiligo patients. J Egypt Womens Dermatol Soc. 2022;19:186-94.
- [CrossRef] [Google Scholar]
- Assessment of acute phase protein serum hs-CRP levels and its role in severity of disease in vitiligo patients-a hospital based case control study in M.Y. hospital, Indore M.P. Int J Contemp Med Res. 2020;7:B1-5.
- [CrossRef] [Google Scholar]
- Serum level of YKL-40, C-reactive protein and ESR in Patients with Vitiligo. Benha J Appl Sci. 2022;7:1-7.
- [CrossRef] [Google Scholar]
- Autoimmunity in Segmental Vitiligo. Front Immunol. 2020;11:568447.
- [CrossRef] [PubMed] [Google Scholar]






