Translate this page into:
The Role of Chemoimmobilization in Improving the Outcome of Scar Revision Surgery
Address for correspondence: Dr Madura Chandraiah, Department of Dermatosurgery, CUTIS Academy of Cutaneous Sciences, 5/1, 4th Main, MRCR Layout, Vijayanagar, Near Veeresh Theatre, Bangalore 560040, India. E-mail: maduradr@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background:
The major goal of scar revision is to make the scar aesthetically more acceptable. The injection of botulinum toxin type A is known to help in improving the outcomes of scars by reducing the tension across the wound edges and by promoting better wound healing.
Objective:
The aim of this article is to evaluate the efficacy of botulinum toxin injection following scar revision surgeries.
Materials and Methods:
A prospective, comparative study involving 20 patients with post-traumatic scars over the face was conducted between July 2018 and July 2019. The patients were divided into two groups: group A underwent scar revision surgery followed by BTX injection on the day of suture removal and group B underwent scar revision surgery alone. The photographic assessment was done at the end of a 1-year follow-up, by three blind investigators based on a pre-designed scale to grade improvement.
Results:
The average grade of improvement of group A (3.02±1.1) was significantly higher when compared with that of group B (2.1±0.8) (P = 0.001).
Conclusion:
This study demonstrates that the combination treatment of scar revision with BTX injection is very effective in producing aesthetically better scars.
Keywords
Botulinum toxin following scar revision
chemoimmobilization of scars
scar revision
INTRODUCTION
Scars can widen when opposing forces that tend to pull at the suture line are applied to newly formed collagen before it reaches final maturity. Such forces can be caused by factors like a muscle pull, elastic forces of adjacent skin, or even an external pressure. Healing may take many months before the scar can mature. Adjuvant treatment like silicone gel sheet, steroids, 5-fluorouracil, scar massage, lasers, and botulinum toxin (BTX) can help improve scars after surgical intervention. Chemoimmobilization with botulinum toxin type A injection is known to help in improving the outcomes of scars by removing the opposing forces exerted by the acting muscles. This temporary paralysis of the acting muscles, which would otherwise interfere with wound healing, gives the crucial advantage of providing rest to the healing wound until the collagen matures.
MATERIALS and METHODS
This is a prospective, comparative study involving 20 patients with a post-traumatic scar over the face, conducted between July 2018 and July 2019. Clearance was obtained from the Institutional Ethics Committee. Patients above the age of 18 years, with facial scars older than 6 months, willing for scar revision surgery and BTX injection, and understood that temporary facial asymmetry could arise because of BTX injection for 4–6 months were enrolled in the study. Patients with keloidal tendency, myasthenia gravis, or any other neuromuscular disorders, those with known allergy to BTX, unable to come down for follow-up visits, and those not ready to accept temporary facial symmetry were excluded. After a detailed history and examination, the patients were recruited and divided into two groups (age- and sex-matched, with scars in similar areas and technique): group A underwent scar revision surgery followed by botulinum toxin type A injection (onabotulinum toxin A—BOTOX (Allergan, Inc., Irvine, CA, USA)) on the day of suture removal and group B underwent scar revision surgery alone. Separate consents for scar revision surgery and botulinum toxin were taken.
The technique of scar revision was decided based on scar morphology, site, orientation with resting skin tension lines, laxity of the surrounding skin, and important functional structures around the scar. All surgeries were performed by a single surgeon under strict aseptic precautions, with atraumatic tissue handling under local infiltrative anesthesia. The standard surgical steps were followed for scar excision and execution of plasties. The undermining was done adequately to just overlap wound edges. All wounds were closed in two layers with intradermal buried absorbable sutures (Vicryl 4.0/5.0) and transcutaneous non-absorbable (Prolene 5.0/6.0) simple interrupted suture. The flaps of W and Z plasty were fixed with half-buried dermal suture with non-absorbable suture (Prolene 6.0). No other technique was performed to reduce mechanical wound tension. The wound was dressed with gentle pressure. Suture removal was done on the 8th day.
Group A patients were injected with botulinum toxin type A on the day of suture removal. The procedure was done under topical anesthesia. The dosage was decided based on the site and length of scar and bulk of underlying muscle which has to be injected. After the suture removal, the patient was asked to animate, and the suture line and immediate surroundings were observed to assess the bulk and direction of the pull of the muscle fibers. The intramuscular injections were given into the expected depth of muscle specific to the anatomic site. The muscles targeted in this study were frontalis, corrugators, procerus, orbicularis oculi, and mentalis. Frontalis muscle received 1–2 U per site approximately at 1 cm distance depending on the length of the scar. Corrugators were injected with 4 U for head and 3 U in the body, and procerus was injected with 6 U. For periorbital scars, orbicularis oculi muscle was injected with 9–12 U in three to four divided doses, and mentalis muscle was given 6 U. In addition to this, BTX was also injected in the 1 cm area surrounding the suture line (perilesional injections) in the intradermal and subcutaneous plane. Intramuscular BTX was given for all scars except the scar on the cheek which received only perilesional injections. Immediate post-procedural ice cube application was done to decrease the reaction of injection prick. They were advised to avoid massaging the area for 48 h. All patients were advised to use Steristrip (applied perpendicular to the wound closure) for 4 weeks, sunscreen topically, and scar massage. They were followed up at 6 weeks, 3 months, 6 months, and 1 year. In each follow-up, scar stretch and color mismatch were assessed with serial photographs [Figure 1A–E].
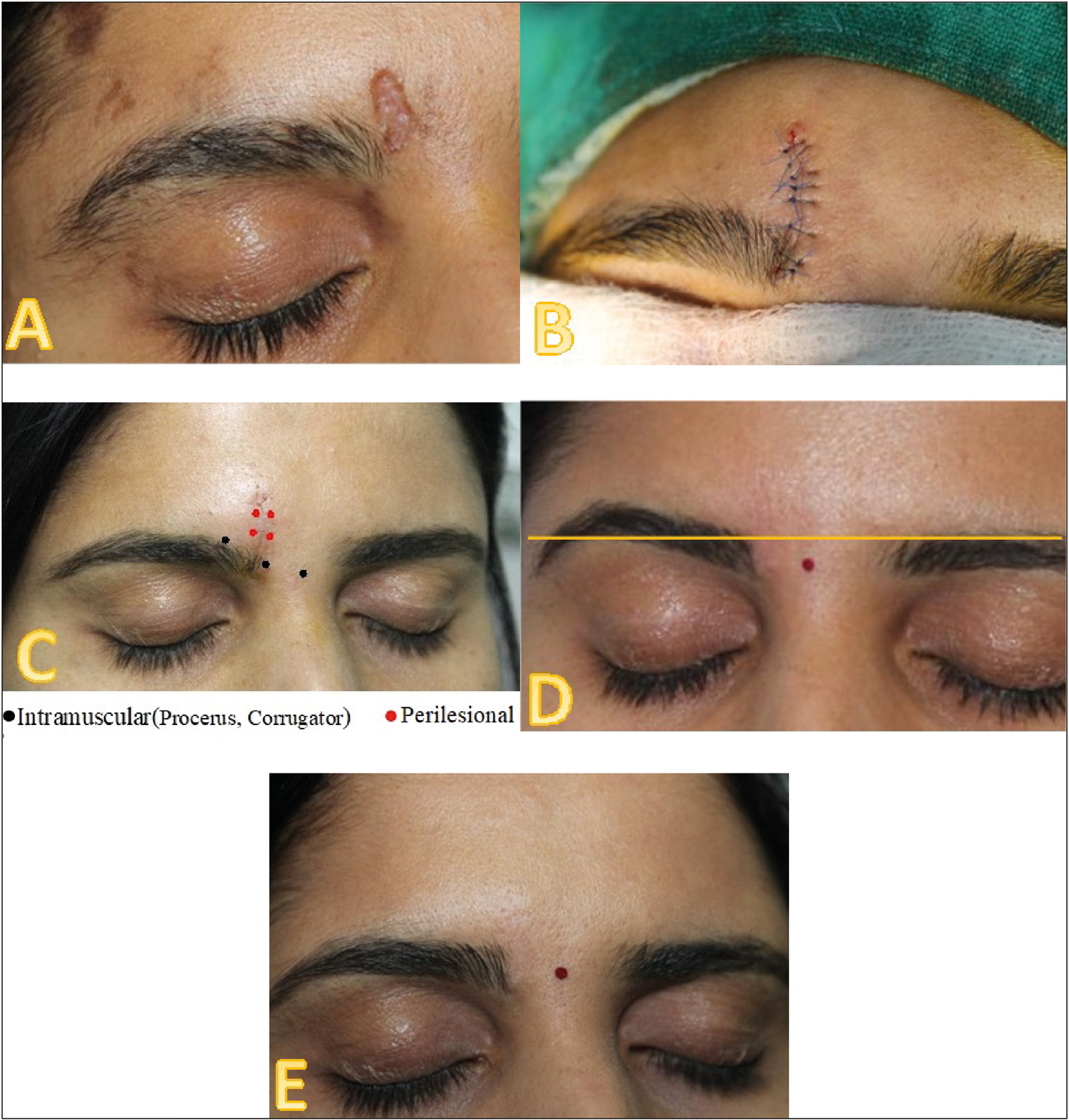
- (A) Patient in group A with a scar over right medical eyebrow—pre-surgical. (B) Immediate post-surgery. (C) At the time of suture removal and injection of BTX. (D) Raised eyebrow following BTX injection. (E) Post-1-year follow-up
All 20 patients completed the study follow-up at 1 year. The photographic assessment was done by three blind investigators, who were involved in neither patient recruitment nor surgery. They subjectively graded the improvement of the scar by comparing the pre- and post-surgical photos, according to the pre-designed scale [Table 1].
| Grade | Degree of improvement |
|---|---|
| −1 | Worsened |
| 0 | No improvement |
| 1 | Mild improvement |
| 2 | Moderate improvement |
| 3 | Good improvement |
| 4 | Excellent improvement |
Statistical analysis was done using the Mann–Whitney U-test calculator to find the significance of parameters between the two groups of patients (P-value <0.05 was considered to be significant).
RESULTS
We enrolled a total of 20 patients, 10 patients into each group for the study. Among them, there were 11 males and 9 females (6 males in group A and 5 males in group B). They were in the age group of 18–38 years. The average age of patients in group A was 28 years and group B was 26 years. Of the 10 scars in each group, six were located over the forehead (four on the central forehead, one on the medial eyebrow, one on left lateral forehead), two over the chin, and one each in the periorbital area and the cheek. The size of the scars ranged from 2 to 6 cm.
Among the 20 scars, 10 were treated with excision and closure, 6 with W-plasty, and 4 with Z-plasty. All patients tolerated the procedure well. The wound healing was uneventful in all patients. In group A, 9 patients received intramuscular and perilesional BTX, whereas 1 patient received only perilesional BTX. The patient with the scar on the medial eyebrow had significant asymmetry of eyebrow due to the unilateral injection of BTX, which was restored to its normal state at the end of 4 months. None of the patients had any additional complications secondary to the diffusion of toxin to surrounding muscles. The effect of BTX had worn off in 4–6 months.
At 1-year follow-up, the average grade of improvement of group A was 3.02±1.1, and group B was 2.1±0.8 and it was statistically significant (P-value being 0.001). In group A, six patients had an average rating of ≥3 and eight patients had an average rating of ≥2. Meanwhile, in group B, two patients had an average rating of ≥3 and six patients had an average rating ≥2 [Figure 2 and Tables 2 and 3].
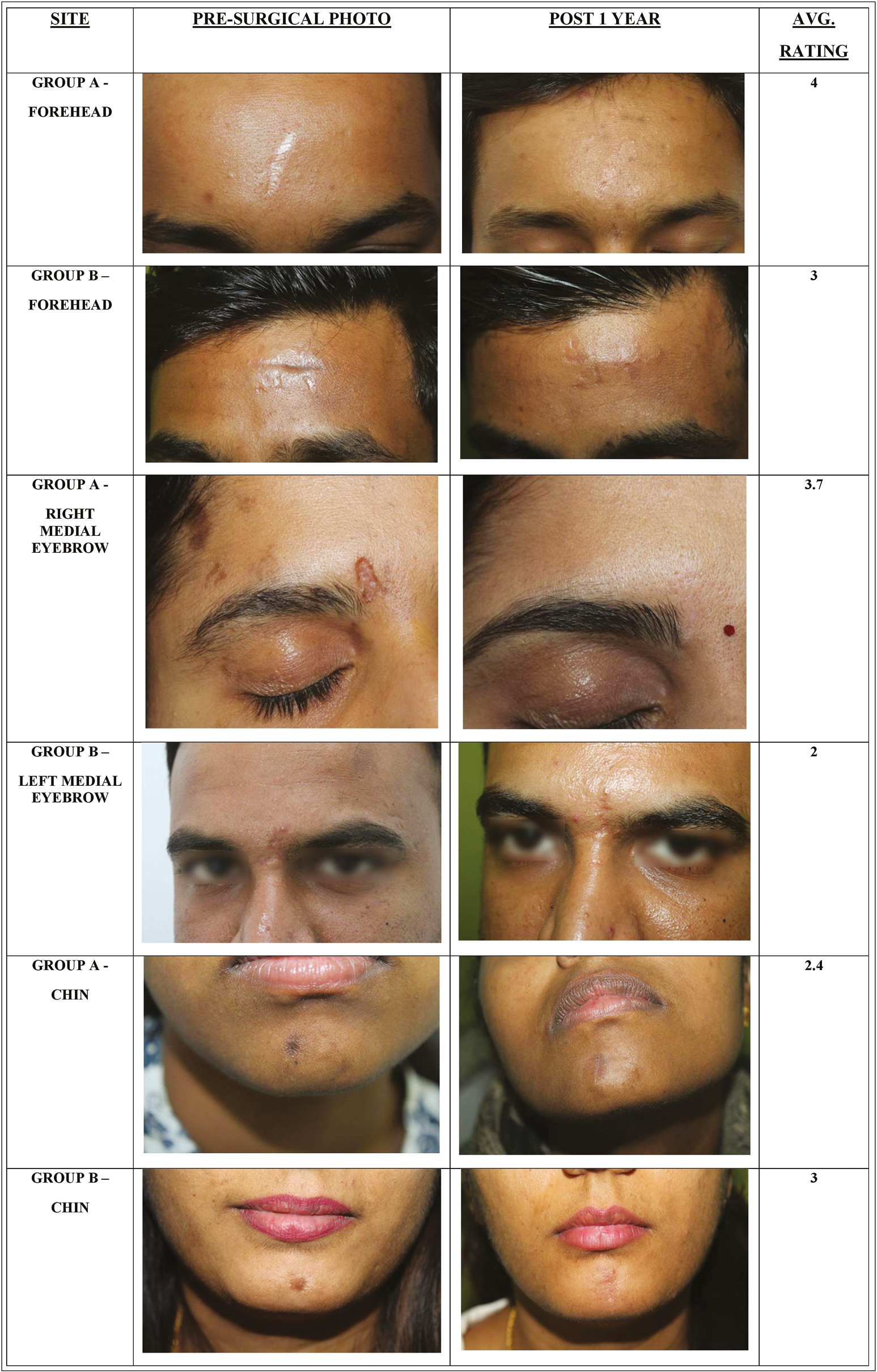
- Pre- and post-surgical photos of three site-matched scars in group A and group B and their average rating
| Patient no. | Site | Length (in cm) | Technique | Btx (in U) | Complications | Avg. rating at 1 year | |
|---|---|---|---|---|---|---|---|
| IM | PL | ||||||
| 1 | Central forehead | 4 | Z plasty | 6 (Frontalis) | 6 | — | 4 |
| 2 | Central forehead | 3 | E&C | 6 (Frontalis) | 4 | — | 3.7 |
| 3 | Central forehead | 3 | E&C | 6 (Frontalis) | 4 | — | 4 |
| 4 | Central forehead | 4 | W plasty | 6 (Frontalis) | 8 | — | 2.7 |
| 5 | Lateral forehead | 4 | Z plasty | 6 (Frontalis) | 8 | — | 1 |
| 6 | Medial forehead | 2 | E & C | 12 (Procerus-5; corrugator head- 4, body-3) | 6 | Asymmetry of the right eyebrow for 4 months | 3.7 |
| 7 | Right cheek | 2.5 | W plasty | 6 (Orbicularis oculi) | 9 | — | 4 |
| 8 | Left cheek | 6 | W plasty | 6 (Orbicularis oculi) | 12 | — | 1.7 |
| 9 | Chin | 3 | E & C | — | 6 | — | 2.4 |
| 10 | Chin | 2.3 | E & C | 6 | 6 | — | 3 |
| Patient no. | Site | Length (in cm) | Technique | Complications | Avg. rating at 1 year |
|---|---|---|---|---|---|
| 1 | Central forehead | 3 | Z plasty | — | 3 |
| 2 | Central forehead | 3 | E&C | — | 1.7 |
| 3 | Central forehead | 2 | E&C | — | 3.4 |
| 4 | Central forehead | 4 | W plasty | — | 2.7 |
| 5 | Lateral forehead | 4 | Z plasty | — | 1.4 |
| 6 | Medial forehead | 2 | E&C | — | 2 |
| 7 | Right cheek | 2.5 | W plasty | — | 2 |
| 8 | Left cheek | 6 | W plasty | — | 1.7 |
| 9 | Chin | 4 | E&C | — | 0.7 |
| 10 | Chin | 2.5 | E&C | — | 2.4 |
DISCUSSION
The major goal of scar revision is to reduce scar visibility and make the scar aesthetically more acceptable. Various techniques such as the W plasty, Z plasty, simple excision and closure, and V-Y plasty are in practice to achieve this goal.
The sutured wound usually gains around 3–7% of tensile strength at 2nd week, 20% of tensile strength at 3rd week, 50% of tensile strength at 4th week, and 80% of tensile strength at 4–6 months.[12] The dynamic musculature of the face is in constant movement and can alter healing following facial surgery. Planning incisions along RSTL, good undermining, and closing wound in layers can help in wound immobilization and minimize the tension in the initial phase of wound healing. The major change in scar remodeling is the conversion of collagen III (immature) to collagen I (mature). During scar remodeling, the contraction of the underlying muscle can exert tension/repeated microtrauma and induce a prolonged inflammatory response and lead to scar widening, hypertrophy, or hyperpigmentation.[3] Scar widening is more for scars aligned perpendicular to Langer’s lines as they are subjected to more repetitive tension. If we can manage the underlying muscle pull on immature collagen, we can prevent scar stretching during these 4–6 months.
One way to eliminate some of the forces that work against wound healing is to decrease tension that is caused by local muscle contraction. Botulinum toxin prevents the release of acetylcholine from the pre-synaptic neurons leading to temporary functional denervation causing clinical flaccid paralysis of the injected muscle. Botulinum toxin-induced temporary paralysis of the muscles that underlie a wound can help minimize tension across wound edges. This was first demonstrated in 2000 on animal models by Gassner et al.[4] in their randomized double-blind placebo-controlled study on primates. Later in 2002, Sherris and Gassner[3] demonstrated this in humans, where they found significant improvement in forehead scars following injection of BTX. It also improved scar formation, particularly during scar revision surgery or during the repair of traumatic lacerations with unfavorable cutaneous orientations.
In our comparative study, we included all cosmetically unacceptable scars (pigmented, depressed, widened) irrespective of their alignment with RSTL. We found that the average grade of improvement of group A (3.02±1.1) was significantly higher than group B (2.1±0.8). This suggests that there was better scar formation in patients who were given BTX injections following scar revision surgery than in patients who underwent scar revision alone.
Ziade et al.[5] in a prospective randomized study of 30 patients with facial wounds had a similar result to our study. Hu et al.[6] in their prospective split scar study of 16 patients injected BTX randomly into half of each surgical wound immediately after surgery and showed better, narrower, and flatter facial scars on the BTX side. In our study, BTX was injected at the time of suture removal. Injection of BTX in the immediate post-operative period was not done in our study as its absorption could be affected in superficial musculature, but it can be an option if only the deeper musculature is being targeted.[7] The authors did not prefer to paralyze the muscle before surgery, as it could affect in the planning of surgery because of immobilization. However, Shome et al.[8] in their prospective study of 100 patients injected BTX 2 weeks before surgery with an intension of preventing any muscular movement around the scar for 2 months post-surgery.
The effect of BTX lasted for 4–6 months. The scars did not worsen after 6 months of follow-up, and that supports the fact that 80% of the tensile strength is achieved by the end of 6 months, although scar remodeling continues until 18 months.
Our study included more of the forehead scars as the functional disability with BTX is less prominent on the forehead when compared with the lower and mid face.[3] The intramuscular dose of BTX was the same as used for other cosmetic indications, whereas the perilesional dose was (5–8 U) dependent on the size of the scar. However, an illustrated case report on lower face wound healing used substantially larger doses of the BTX than for cosmetic purposes to eliminate the complete dynamic tension which resulted in expected functional disability.[7] Choi et al.[9] injected cosmetic doses of BTX following eyelid reconstruction and found improved wound scarring. Chang et al.[10] injected 0.1 mL of BTX at 6 points, immediately after skin closure to orbicularis oris muscle post cleft lip repair, and had significant improvement in scars.
Mechanical forces such as stretch, tension, shear forces, and pressure are perceived as a stimulus to cell proliferation, angiogenesis, and epithelialization and are modulated by transforming growth factor (TGF-β). These mechanical stimuli are received by mechanosensitive nociceptors, and activation of these leads to the production of neuropeptides that provoke proinflammatory response.[11] Jeong et al.[12] in 2015 found that BTX directly inhibits fibroblast-to-myofibroblast differentiation in vitro. Apart from its tension-relieving properties, BTX has an inhibitory effect on fibroblasts and TGF-β. Thus, perilesional injection into areas adjacent to the suture line will form a better scar and may also prevent a hypertrophic scar. In our study, one patient received only perilesional BTX injection (average rating—2.4).
Overall, in group A, we had six patients with an average rating of ≥3 and eight patients had an average rating of ≥2. In group B, two patients had an average rating of ≥3 and six patients had an average rating of ≥2. Hence, both therein work in improving scar.
Post-procedure, both groups were advised scar massage and also to apply Steristrips,[13] perpendicular to the direction of wound closure. This can reduce the tensile distracting force of elastic forces of adjacent skin. Our patients were compliant with the minimal facial asymmetry for 3–6 months, which was explained to them before the procedure.
This study included patients of only scar revision, wherein all the rules to reduce the mechanical tension were followed to achieve superior results which helped to prove that botulinum toxin A further improves the scar mechanics and outcome especially for wound perpendicular to Langer’s lines. The only downside of BTX injection following scar surgeries is it adds to the additional cost of surgery.
The limitations of our study are the small sample size, lack of randomization while recruiting patients, and the treatment results being compared on different patients. Though it is age-, sex-, and technique-matched controls with scars in similar areas, the interpatient wound healing differences which have genetic influence cannot be explained.
CONCLUSION
The surgical scar revision is a well-established technique for dermatologic surgeons. BTX provides rest to the healing wounds until the completion of collagen remodeling. Hence, the BTX injection is one of the adjuvant therapies and this study strongly suggests that a combination of scar revision with BTX injection is very effective to obtain excellent results.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74:589-605.
- [Google Scholar]
- Prediction of wound tensile strength: An experimental study. Br J Surg. 1992;79:401-3.
- [Google Scholar]
- Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg. 2000;105:1948-53; discussion 1954-5.
- [Google Scholar]
- Use of botulinum toxin type A to improve treatment of facial wounds: A prospective randomised study. J Plast Reconstr Aesthet Surg. 2013;66:209-14.
- [Google Scholar]
- Effects of botulinum toxin on improving facial surgical scars: A prospective, split-scar, double-blind, randomized controlled trial. Plast Reconstr Surg. 2018;141:646-50.
- [Google Scholar]
- Botulinum toxin-induced immobilization of lower facial wounds. Arch Facial Plast Surg. 2009;11:140-2.
- [Google Scholar]
- An algorithm using botox injections for facial scar improvement in Fitzpatrick type IV-VI skin. Plast Reconstr Surg Glob Open. 2018;6:e1888.
- [Google Scholar]
- Use of botulinum A toxin inpatients at risk of wound complications following eyelid reconstruction. Ophthal Plast Reconstr Surg. 1997;13:259e64.
- [Google Scholar]
- Botulinum toxin to improve results in cleft lip repair: A double-blinded, randomized, vehicle-controlled clinical trial. PLoS One. 2014;9:e115690.
- [Google Scholar]
- Scar prevention and remodeling: A review of the medical, surgical, topical and light treatment approaches. Int J Dermatol. 2014;53:922-36.
- [Google Scholar]
- Effect of botulinum toxin type A on differentiation of fibroblasts derived from scar tissue. Plast Reconstr Surg. 2015;136:171e-8e.
- [Google Scholar]
- Current methods employed in the prevention and minimization of surgical scars. Dermatol Surg. 2011;37:1740-6.
- [Google Scholar]






