Translate this page into:
A Simplified Reconstructive Technique for Full-thickness Central Defects of the Auricle with the Use of a Post-auricular Folded Flap
Address for correspondence: Dr. Ioannis Papadiochos, Department of Oral and Maxillofacial Surgery, Theagenio Cancer Hospital, Thessaloniki, Greece. E-mail: andricarus@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Post-auricular flaps have proved very effective in the reconstruction of most types of partial auricular defects. However, few reports exist regarding the reconstruction of centrally located perforating defects of the auricle.
Objectives:
This paper aimed to describe a one-stage technique including a post-auricular folded flap (PAFF) for reconstruction of full-thickness defects of central auricular sites.
Patients and Methods:
Between March 2010 and November 2014, five male patients were treated with this reconstructive technique under local anaesthesia. At the time of surgery, patients' age ranged from 76 to 86 years (mean age, 79.8). The patients suffered from a central and full-thickness defect owing to surgical excision of a skin malignancy or failed reconstruction procedures secondary to surgical excision of a skin malignancy.
Results:
Healing was uneventful for all the included patients, without signs of dehiscence, necrosis, hematoma and infection. The defects were completely repaired, without the need of further operations. During the follow-up period, all the patients remained satisfied with the aesthetic outcome.
Conclusions:
This technique constitutes an immediate, effective and low-morbidity procedure to repair full-thickness central defects of the auricle. Since PAFF requires only one surgical operation under local anaesthesia, patients with burdened medical history may profit from this technique.
Keywords
Auricle reconstruction
central defects
full-thickness
post-auricular flap
INTRODUCTION
Reconstruction of auricular defects always remains a very demanding and challenging procedure. Various techniques have been developed depending on the defect size, site and type of tissues involved. Irrespective of the selected technique, auricular reconstruction should aim at deformity correction with the most minimal morbidity as well as the accomplishment of the most pleasing aesthetic outcome. On reviewing the literature, there is little evidence regarding reconstruction of centrally located and full-thickness defects.[1]
Aims
The aim of this article was to describe in detail the post-auricular folded flap (PAFF), which was utilised for reconstruction of perforating defects of central sites of the auricle in elderly patients with advanced systemic diseases.
PATIENTS AND METHODS
Surgical technique
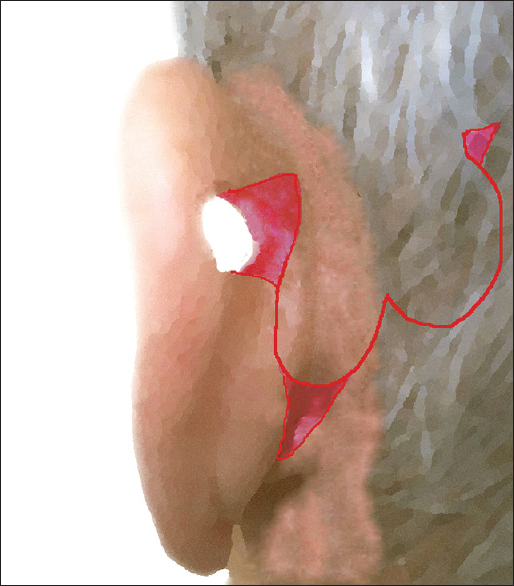
The patient should be positioned and prepped in the similar fashion as for otologic surgery. Thus, supine position is selected, with the head slightly elevated to limit blood loss. PAFF represents an inferiorly based myocutaneous transpositional flap; its base was approximately placed inferiorly or at the same height to the defect, and its long axis was drawn parallel to the auriculocephalic sulcus (ACS). The flap width is marked to be equal with maximal horizontal dimension of defect; its maximum length should be approximately 3–4 times greater of its width. Indeed, the flap length needs to be large enough to allow its distal half to be comfortably folded and cover the defect with adequate amount of tissue in both auricular aspects. Furthermore, a small skin area of medial auricular surface between the defect and flap, with rectangular shape, is marked. This area is used to accommodate the transpositioned proximal part of PAFF, after de-epithelialisation.
Once the outline of flap is defined [Figure 1a], its elevation initiates under local anaesthesia (mepivacaine chlorohydrate with adrenaline 1:100,000) with an incision extending to periosteum and to auricular pericondrium. This facilitates incorporation of local muscular fibres as well as branches of the post-auricular artery (PAA) and local nerves. Provided that PAFF may be used to reconstruct an already healed defect, the margins of the latter are properly de-epithelialised and undermined. When the flap is appropriately raised, the de-epithelialisation of rectangular area intervenes, and it is transpositioned to the lateral auricular surface through the defect [Figure 1b and c]. Afterwards, its distal half is properly folded, orientated and rolled up in a manner that the defect is covered with skin in both aspects (medial and lateral). A 3–4 mm zone of the flap, which curves to form the fold, is de-epithelialised to enable approximation and suspension with interrupted sutures from the also de-epithelialised defect margins [Figure 2a and b]. The proximal part of PAFF is seated on the medial rectangular area and is sutured too. After adequate skin undermining, interrupted sutures were used to close the donor site by primary intention, without tension [Figure 2c]. No extra incisions or grafts were required for donor site closure.
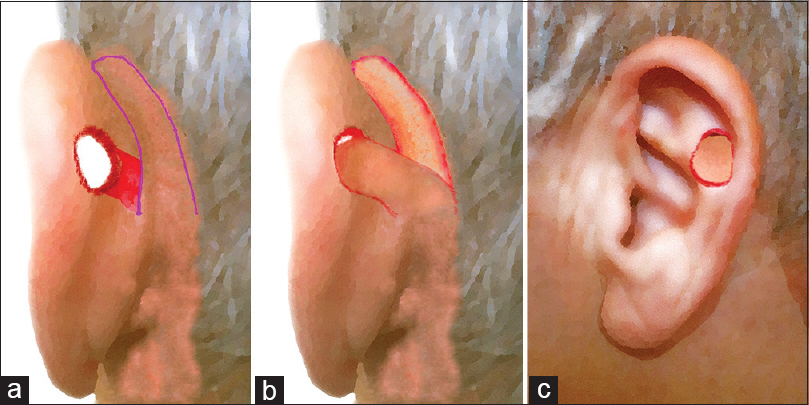
- Schematic illustration of the operative steps: (a) The design of flap; the red small area of medial surface between the defect and flap, with rectangular shape, is marked. (b and c) The flap transpositioned to the lateral auricular surface through the defect
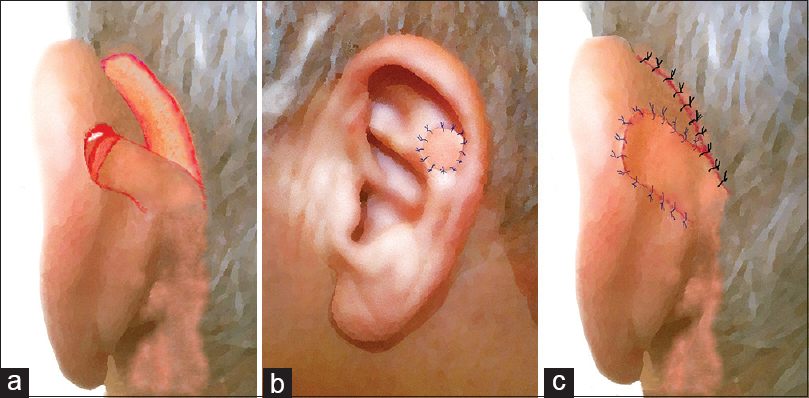
- Schematic illustration of the operative steps: (a and b) A 3–4 mm zone of the flap forming the fold, is de-epithelised to enable flap's approximation and suspension with sutures from the also de-epithelialised defect margins. (c) The proximal part of post-auricular folded flap is seated on the medial rectangular area and is sutured too. Interrupted sutures were used to close the donor site by primary intention
Clinical study
An 83-year-old male patient presented to our outpatient department to repair a full-thickness elliptical defect on his left auricle. The size of the defect was measured 1.2 cm × 0.8 cm and was located on central portion of the antihelix [Figure 3a]. This deformity occurred as result of a previous failed reconstruction with free skin graft after basal cell carcinoma excision. Under local anaesthesia, an inferiorly based transpositional flap from the post-auricular region was raised, properly folded and sutured [Figure 3b–d]. The vascular supply of the flap was derived from the PAA. The wound at the donor site was closed by primary intention [Figure 3e]. Mild venous congestion lasted only for the first 2 days [Figure 3f]. The standard care comprised a prophylactic antibiotic protocol with the administration of an intravenous dose of cefuroxime 30 min before the operation. Postoperatively, a dressing (paraffin gauze) containing sodium fusidate 2% covered the surgical sites and a pressure bandage remained in place for around 24 h. Oral antibiotics were also prescribed and patients were instructed to apply 2% mupirocin ointment three times daily for 5 days. The sutures were removed 8–10 days postoperatively.
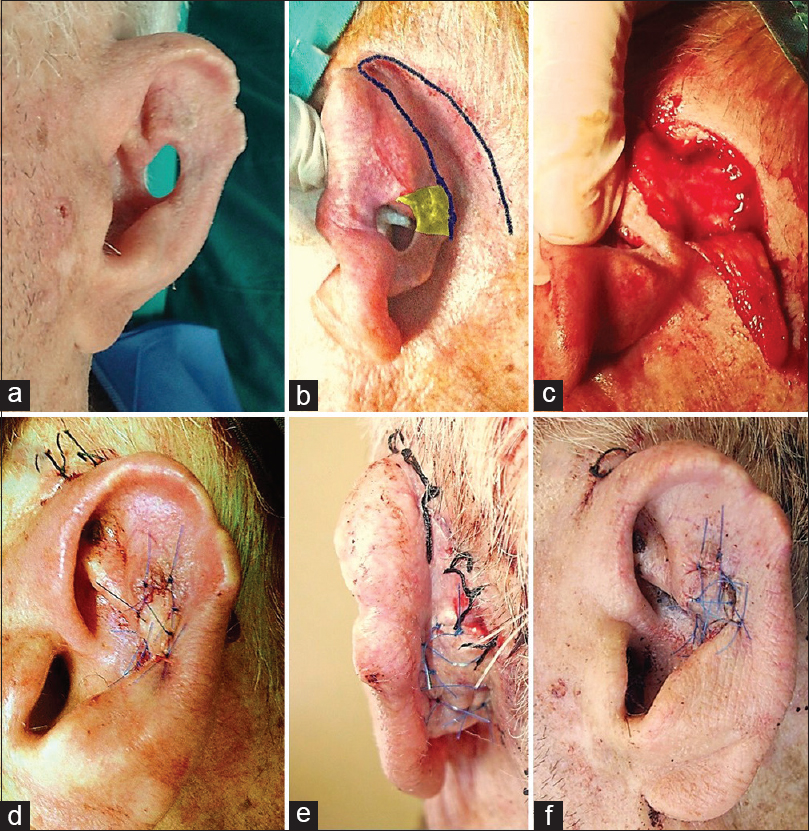
- (a-e) Stages of reconstruction with post-auricular folded flap in 83-year-old patient. Yellow area illustrates the medial rectangular area needing de-epithelialisation.(f) Mild venous congestion 20 h postoperatively
Between March 2010 and November 2014, a total of five male patients were operated on with this reconstructive technique. At the time of surgery, patients' ages ranged from 76 to 86 years (mean age, 79.8; median age, 78 years). All patients suffered from a both non-helical and full-thickness defect with maximal diameter ranging between 1.1 and 1.4 cm as a result of either surgical excision of a skin malignancy (n = 3) or failed reconstruction (skin graft, local flaps and primary closure). Before reconstruction there was histologic confirmation of no residual disease. The patients with a history of failed reconstruction (n = 2) remained disease-free for at least 6 months. Three patients were classified in Category III and two in Category IV, according to the American Society of Anesthesiologists physical status classification system.[2] None of the patients had undergone ipsilateral parotid surgery, neck dissection and surgery of retro- and post-auricular region. All patients operated under local anaesthesia and were discharged on the same or following day of the surgery.
RESULTS
Follow-up period ranged from 19 to 42 months (median, 29 months; mean, 28.4 months). Mild venous congestion occurred in two flaps, but it resolved in both after 2 days. A small area of ecchymosis occurred in one patient in the mastoid area, without affecting the healing process. Complications such as partial or total flap necrosis, formation hematoma, haemorrhage, suture granuloma, local tumor recurrence and wound infection or dehiscence were not observed. Satisfactory colour match was achieved with all flaps, and besides, theirs skin sensibility was preserved to various degrees. Neither reduction in auricle height nor shape distortion occurred in any of the patients. The donor site scars were placed on ACS and proved inconspicuous. However, the pin-cushioning or trapdoor phenomenon was obvious in all patients, especially in medial aspects of their auricles. Slight reduction of auriculocephalic angle was noted in two patients in immediate post-operative period, but without obvious asymmetry with the contralateral auricle during follow-ups. All patients declared highly satisfied with the final appearance, since the outcome fulfilled their aesthetic requirements [Figure 4].
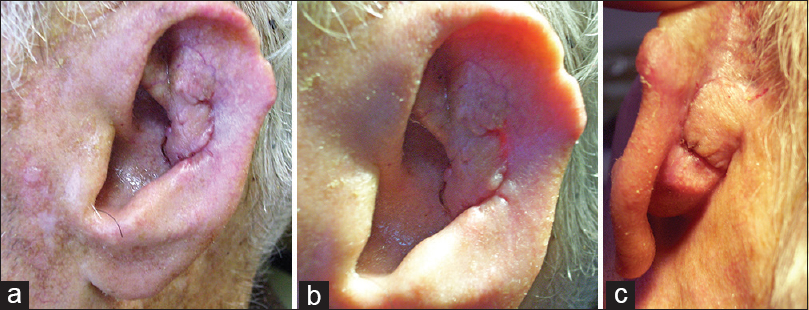
- (a-c) Clinical views of the repaired defect, 6 weeks after surgery. The pin-cushioning or trapdoor phenomenon is apparent in both aspects
DISCUSSION
The post-auricular area is regarded as valuable donor site for harvesting skin grafts for local, regional and free flaps for head and neck reconstruction.[34] This area provides several advantages such as abundant vascularisation, minimal morbidity, vicinity to the auricular defects, feasibility for direct closure of donor site, well-concealed scars in the ACS and good skin match to head and neck subunits.[35] Furthermore, this area provides the potentiality for raising innervated flaps with preservation of the cutaneous branches of the great auricular nerve.[67]
In terms of surgical anatomy, the rich blood supply, which characterises the skin of the post-auricular area, originates from an arterial arcade created by the anastomosis between the auricular branch of the PAA and posterior branch of the superficial temporal artery.[8] This arcade lies in the ACS, deeper to the post-auricular muscle, between the auricular cartilage and the periosteum of temporal bone.[8] Overall, the skin behind the auricle accepts rich blood supply from many directions thanks to dense superficial and deep dermal plexuses.[8] Both the posterior branch of great auricular nerve and the lesser occipital nerve are responsible for the nerve supply skin of post-auricular area, and their nerve fibers course 3–5 mm below the skin surface.[69]
For many decades, a great variety of modifications for harvesting retro- or post-auricular flaps have been described under various terminologies.[89] Different sites and types of defects correspond to different designs for reconstruction with a post-auricular flap. However, since perforating defects of central auricular region are less commonly observed, there is paucity of publications involved with their reconstruction.[11011] In fact, many uses of post-auricular flaps pertain to central cartilage-exposing defects.[12131415]
Heinz et al. introduced the anterior pedicled retroauricular flap for the reconstruction of such defects, which encompassed three surgical steps.[1] The same authors later amended this technique, adding a cranial pedicled preauricular flap to obviate one surgical stage.[10] PAFF comprises only one surgical intervention which is advantageous for patients with burdened medical history. The rich blood supply, pliability and thickness of PAFF allow for surgical manipulations such as fold and de-epithelialisation without jeopardising the risk of ischemic necrosis. De-epithelialisation is a common element in stepped procedures of post-auricular flaps and is safely performed.[8131416] The adequate thickness of PAFF not only achieves excellent defect coverage in both auricular aspects, but also eliminates the need for cartilage grafts and other complex flap harvesting procedures. In contrast to chondrocutaneous advancement flap reported by Ramirez and Heckler,[17] PAFF produced neither reduction of auricle size nor distortion of its shape. Although post-auricular flaps can be designed in a random pattern,[8] PAFF was harvested as an inferiorly based axial flap, nourished by branches from PAA and incorporating the supportive muscle. Myocutaneous flaps based on the PAA have been successfully applied in auricular reconstruction.[18192021]
PAFF shares some similarities with the transposition-rotation bilobed flap (TRBF) which introduced by Weerda and Münker,[22] for reconstruction of full-thickness helical and non-helical defects [Figure 5]. The longer/primary lobe of the TRBF is folded and after proper de-epithelialisation, is suspended from the defect margins in identical manner with the PAFF. A small rectangular area in medial auricular surface is de-epithialised too. Nevertheless, the TRBF requires extended dissection and circumferential undermine, while the primary closure of its donor site may need extra incisions or removal of Burrow's triangles. Despite its large base and the potentiality for closing bigger defects in comparison to PAFF, its superiorly based design impinges the ascending branches of local nerves and the pedicle of PAA, which are consequently severed.
- The transposition-rotation bilobed flap
CONCLUSIONS
In summary, the PAFF provides to facial reconstructive surgeons a reliable option to reconstruct full-thickness defects of the central auricle with low morbidity and satisfactory outcomes in one surgical intervention, which may be performed under local anaesthesia on an outpatient basis. However, this retrospective case-series study contained very low number of cases and all the defects had relatively small size.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Repairing a non-marginal full-thickness auricular defect using a reversed flap from the postauricular area. J Oral Maxillofac Surg. 2015;73:764-8.
- [Google Scholar]
- American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011;55:111-5.
- [Google Scholar]
- Retroauricular skin: A flaps bank for ear reconstruction. J Plast Reconstr Aesthet Surg. 2008;61(Suppl 1):S44-51.
- [Google Scholar]
- Retroauricular flap: Its clinical application and safety. Br J Plast Surg. 2001;54:12-9.
- [Google Scholar]
- The posteriorauricular-auricular flap. In: Sarafin D, ed. Atlas of Microsurgical Composite Tissue Transplantation. Philadelphia: W. B. Saunders Company; 1996.
- [Google Scholar]
- An innervated retroauricular skin flap for total earlobule reconstruction. Br J Plast Surg. 2003;56:818-21.
- [Google Scholar]
- Reconstruction of the anterior surface of the ear using a postauricular pull-through neurovascular island flap. Ann Plast Surg. 2006;56:609-13.
- [Google Scholar]
- Versatility of the posterior auricular flap in partial ear reconstruction. Plast Reconstr Surg. 2010;126:1213-21.
- [Google Scholar]
- Two-step reconstruction of non-marginal auricular defects. J Oral Maxillofac Surg. 2016;74:1494-8.
- [Google Scholar]
- Ear reconstruction with rotation-advancement composite flap. Plast Reconstr Surg. 1985;75:567-8.
- [Google Scholar]
- Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-7.
- [Google Scholar]
- The trap door flap: A reliable, reproducible method of anterior pinna reconstruction. J Plast Reconstr Aesthet Surg. 2013;66:1360-4.
- [Google Scholar]
- Reconstruction of anterior auricular conchal defect after malignancy excision: Evolving-door flap versus full-thickness skin graft. J Plast Reconstr Aesthet Surg. 2010;63:746-52.
- [Google Scholar]
- Retroauricular pull-through island flap for defect closure of auricular scapha defects – a safe one-stage technique. J Plast Reconstr Aesthet Surg. 2011;64:934-6.
- [Google Scholar]
- Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:e219-20.
- [Google Scholar]
- Reconstruction of nonmarginal defects of the ear with chondrocutaneous advancement flaps. Plast Reconstr Surg. 1989;84:32-40.
- [Google Scholar]
- How I do it – otology and neurotology. A specific issue and its solution. A postauricular muscle-skin flap for conchal defects. Laryngoscope. 1982;92:596-8.
- [Google Scholar]
- Auricular reconstruction using postauricular myocutaneous flap. Laryngoscope. 1994;104(6 Pt 1):778-80.
- [Google Scholar]
- Auricular reconstruction with postauricular myocutaneous flap. Otolaryngol Head Neck Surg. 1983;91:193-6.
- [Google Scholar]
- Auricular reconstruction with a postauricular myocutaneous island flap: flip-flop flap. Plast Reconstr Surg. 1996;98:1191-9.
- [Google Scholar]
- The “transposition-rotation flap” in the one stage reconstruction of auricle defects (author's transl) Laryngol Rhinol Otol (Stuttg). 1981;60:312-7.
- [Google Scholar]






